Transmission
COVID-19 and other respiratory viruses are spread mainly through close person-to-person contact (between people who are physically near each other [<6 ft]). Pre-symptomatic and asymptomatic carriers can also spread the virus.
COVID-19 spreads very easily from person to person
- The Omicron COVID-19 variant is more contagious than influenza and perhaps as contagious as measles, which is among the most contagious human respiratory viruses.
- Infections occur mainly through exposure to respiratory droplets when a person is in close contact with someone who has COVID-19. Respiratory droplets cause infection when they are inhaled or deposited on mucous membranes, such as those that line the inside of the nose and mouth.
- Some infections can be spread by exposure to the virus in small droplets and particles that can linger in the air for minutes to hours. These viruses may be able to infect people who are more than 6 feet away from the person who is infected or after that person has left the space. The risk is greater within enclosed spaces with inadequate ventilation.
COVID-19 spreads less commonly through contact with contaminated surfaces
- Contamination of surfaces with droplets of the COVID-19 virus is possible and may persist for hours and, potentially, days. (Omicron can persist for up to 194 hours on plastic and 21 hours on skin.)
- Persistence is greater on nonporous surfaces and at room temperature rather than warmer temperatures.
- Recommendations include decontaminating surfaces, especially those that have high utilization and contact frequency (eg, counters, tabletops, doorknobs, keyboards, tablets); performing frequent hand hygiene; and avoiding touching the face.
- COVID-19 and other respiratory viruses are killed by standard cleaning solutions.
COVID-19 spreads between people and animals
- COVID-19 can infect many mammals, including companion animals (cats, dogs, hamsters, and ferrets), several types of zoo animals, and wildlife (including deer, otters, and mink).
- However, the risk of COVID-19 spreading from animals to people is low.
More information can be found from the CDC’s “How COVID-19 Spreads.”
Metered-dose inhalers versus nebulization
Author: Mark Hauswald, MS, MD, FACEP, Emeritus Professor of Emergency Medicine, Past Associate Dean for Clinical Affairs and Patient Safety, Past Director of Global Health Projects, University of New Mexico Health Sciences Center; and Susan R. Wilcox, MD, FACEP, Division Chief, Critical Care, Department of Emergency Medicine, Associate Professor of Emergency Medicine, Harvard Medical School, Associate Chief Medical Officer, Boston MedFlight
Nebulized medications should be avoided because they can aerosolize the virus and increase the risk of exposure for health care workers and other patients. Respiratory medications should be administered as metered-dose inhalers.
BiPAP and CPAP
Author: Mark Hauswald, MS, MD, FACEP, Emeritus Professor of Emergency Medicine, Past Associate Dean for Clinical Affairs and Patient Safety, Past Director of Global Health Projects, University of New Mexico Health Sciences Center; and Susan R. Wilcox, MD, FACEP, Division Chief, Critical Care, Department of Emergency Medicine, Associate Professor of Emergency Medicine, Harvard Medical School, Associate Chief Medical Officer, Boston MedFlight
Both BiPAP and CPAP are controversial in patients with COVID-19. They can help patients avoid being intubated in some cases and can help better allocate scarce ventilators. However, the concern is that these noninvasive positive-pressure ventilation (NIPPV) methods could, theoretically, increase aerosolization of the virus and the exposure risk to people nearby. Additionally, experience with prior viral pneumonias demonstrated a high treatment failure rate of approximately 60% to 80% with these methods. Lastly, there are concerns that patients with acute respiratory distress syndrome (ARDS) may have large, unchecked tidal volumes on NIPPV, which would lead to lung-injurious ventilation. Although many institutions do not promote NIPPV in patients with COVID-19, its use has been supported by the Society of Critical Care Medicine, with caveats. If NIPPV is used, patients must be monitored carefully, and intubation should not be delayed if any signs of worsening occur. However, there is insufficient evidence to recommend CPAP or BiPAP.
High-flow nasal oxygen
Article summary: respiratory support for adult patients with COVID‐19
Whittle JS, Pavlov I, Sacchetti AD, Atwood C, Rosenberg MS. Respiratory support for adult patients with COVID-19. JACEP Open. 2020 Apr 2;1(2):95-101. doi:10.1002/emp2.12071
Author: Jessica Whittle, MD, PhD, FACEP, Professor and Vice Chair of Research, Department of Emergency Medicine, University of Tennessee College of Medicine
High-flow nasal oxygen (HFNO) includes a variety of devices that provide heated, humidified oxygen at flow rates up to 80 L/min. These devices use either large-bore or small-bore nasal cannulas. For any given flow rate, large-bore nasal cannulas generate lower velocity gas compared to small-bore cannulas. These differences mean that device settings are not universal and clinicians should titrate to effect.
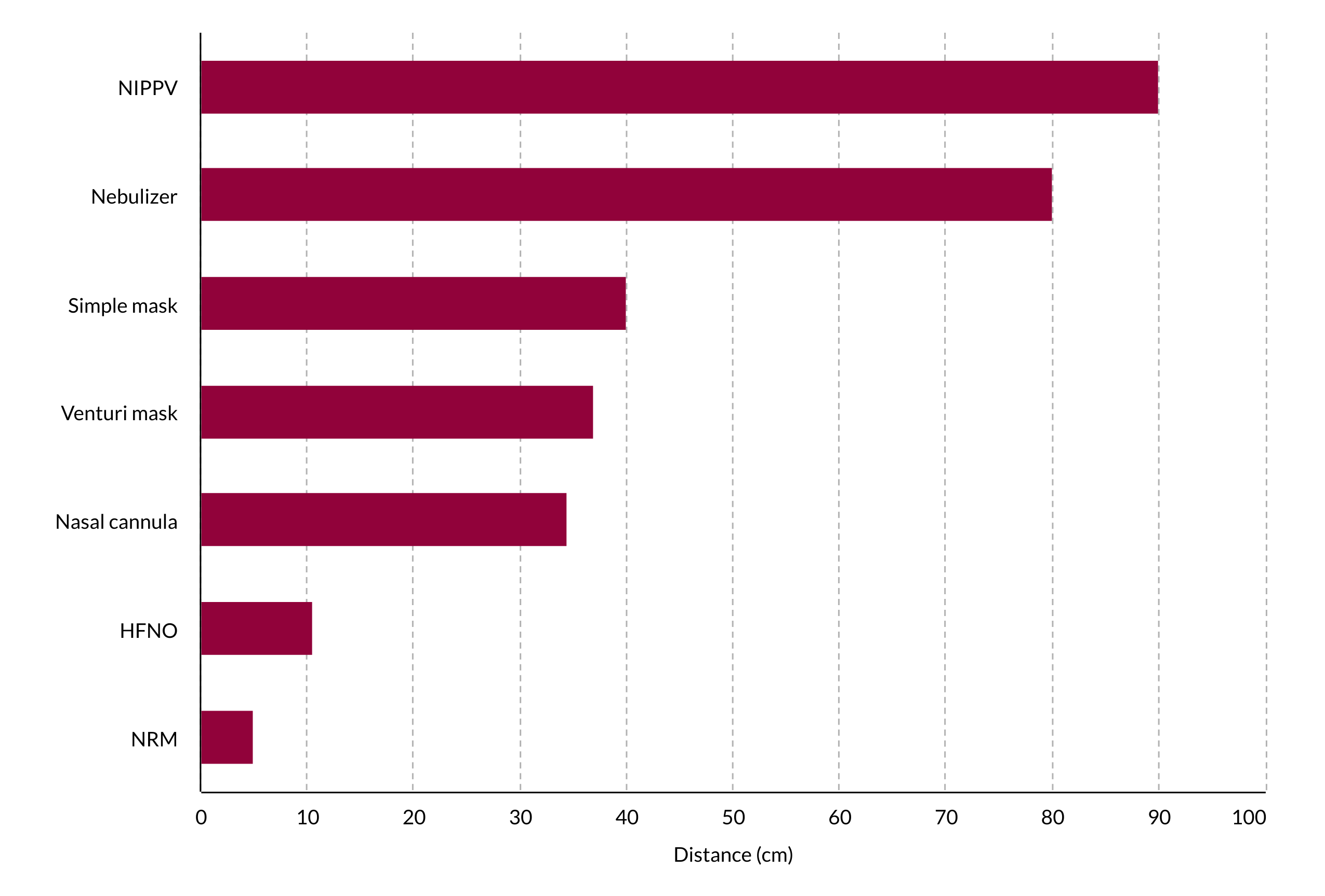
The use of HFNO has been associated with lower mortality in hypoxemic respiratory failure.1 Compared to conventional oxygen therapy, HFNO is associated with a decreased risk of subsequent intubation (RR 0.85; 95% CI, 0.74-0.99) and need for ICU admission.2-4 Concern about aerosolization with HFNO has led to recommendations against it. However, aerosolization with HFNO is minimal, and it is now recommended as the oxygenation therapy of choice in patients with respiratory distress (Figure 3.3). Guidelines from the WHO, the Italian Thoracic Society, the respiratory care committee of the Chinese Thoracic Society, and the Australian and New Zealand Intensive Care Society; a joint statement from the German intensive care, anesthesia, and emergency medicine societies; and the joint guidelines produced by the European Society of Intensive Care Medicine and the Society of Critical Care Medicine all recommend HFNO as a therapy for COVID-19 respiratory failure.5-10 Recent publications suggest that newer HFNO and NIPPV systems with good interface fitting do not create widespread dispersion of exhaled air and, therefore, may be associated with low risk of airborne transmissions.5,8
Placing a surgical mask over patients with potential airborne infections like COVID-19 during high-flow therapies is recommended.9 High-fidelity human mannequin simulation studies showed that surgical masks reduce exhaled air dispersion.11 Computational fluid dynamic simulation also demonstrated that a patient with a surgical mask who was treated with HFNO at maximal settings generated an aerosol dispersion cloud similar to a patient with tidal breathing.12 Staff should use airborne protections, and patients should be treated in a negative-pressure room, if available.5,8
No currently published evidence supports that HFNO is a risk factor for nosocomial transmission of respiratory pathogens.13-16 During the 2003 Toronto SARS-CoV outbreak, HFNO was not found to be a risk factor for transmission to health care workers; endotracheal intubation, by contrast, was strongly associated with transmission to health care workers.17
Currently, there are no well-accepted criteria for HFNO failure, but patients who require vasopressor support and whose respiratory rate and thoracoabdominal asynchrony are not rapidly relieved with HFNO are potentially at high risk of HFNO failure.16,18 The ROX index, the ratio of oxygen saturation to respiratory rate, can be used to monitor therapy and aids in predicting clinical outcomes of patients treated with HFNO. A ROX greater than 4.88 is predictive of success, meaning the patient is unlikely to progress to mechanical ventilation.19 A subsequent meta-analysis of 13 studies supported the use of the ROX index but suggested that the optimal time for measurement and the most valuable prognostic cutoff remain unclear.20 Patients with established acute respiratory distress syndrome (ARDS) should move rapidly to mechanical ventilation and be treated per published recommendations.21,22
Figure 3.3 Comparison of aerosol dispersion differences using various treatment modalities. Credit: ACEP.
References
- Frat JP, Thille AW, Mercat A, et al; FLORALI Study Group; REVA Network. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015 Jun A4;372(23):2185-2196. doi:10.1056/NEJMoa1503326
- Rochwerg B, Granton D, Wang DX, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019 May;45(5):563-572. doi:10.1007/s00134-019-05590-5
- Nagata K, Morimoto T, Fujimoto D, et al. Efficacy of high-flow nasal cannula therapy in acute hypoxemic respiratory failure: decreased use of mechanical ventilation. Respir Care. 2015 Oct;60(10):1390-1396. doi:10.4187/respcare.04026
- Plate JDJ, Leenen LPH, Platenkamp M, Meijer J, Hietbrink F. Introducing high-flow nasal cannula oxygen therapy at the intermediate care unit: expanding the range of supportive pulmonary care. Trauma Surg Acute Care Open. 2018 Aug 3;3(1):e000179. doi:10.1136/tsaco-2018-000179
- Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. WHO. Published January 28, 2020.
- Harari SA, Vitacca M, Blasi F, Centanni S, Santus PA, Tarsia P. Managing the Respiratory Care of Patients With COVID-19. Italian Thoracic Society - Associazione Italiana Pneumologi Ospedalieri - Societa Italiana Di Pneumologia; 2020.
- Respiratory care committee of Chinese Thoracic Society. Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020 Feb;17(0):E020. doi:10.3760/cma.j.issn.1001-0939.2020.0020
- ANZICS COVID-19 Guidelines. The Australian and New Zealand Intensive Care Society; 2021.
- Kluge S, Janssens U, Welte T, Weber-Carstens S, Marx G, Karagiannidis C. German recommendations for critically ill patients with COVID-19. Med Klin Intensivmed Notfmed. 2020 Dec;115(suppl 3):111-114. doi: 10.1007/s00063-020-00689-w.
- Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020 May;46(5):854-887. doi:10.1007/s00134-020-06022-5
- Hui DS, Chow BK, Chu L, et al. Exhaled air dispersion during coughing with and without wearing a surgical or N95 mask. PLoS One. 2012;7(12):e50845. doi:10.1371/journal.pone.0050845
- Leonard S, Atwood CW, Walsh BK, et. al. Preliminary findings on control of dispersion of aerosols and droplets during high-velocity nasal insufflation therapy using a simple surgical mask: implications for the high-flow nasal cannula. Chest. 2020 Sep;158(3):1046-1049. doi: 10.1016/j.chest.2020.03.043
- Leung CCH, Joynt GM, Gomersall CD, et al. Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: a randomized controlled crossover trial. J Hosp Infect. 2019 Jan;101(1):84-87. doi:10.1016/j.jhin.2018.10.007
- Kotoda M, Hishiyama S, Mitsui K, et al. Assessment of the potential for pathogen dispersal during high-flow nasal therapy. J Hosp Infect. 2020 Apr;104(4):534-537. doi:10.1016/j.jhin.2019.11.010
- Raboud J, Shigayeva A, McGeer A, et al. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLoS One. 2010 May 19;5(5):e10717. doi:10.1371/journal.pone.0010717
- Rello J, Perez M, Roca O, et al. High-flow nasal therapy in adults with severe acute respiratory infection: a cohort study in patients with 2009 influenza A/H1N1v. J Crit Care. 2012 Oct;27(5):434-439. doi:10.1016/j.jcrc.2012.04.006
- Bouadma L, Lescure FX, Lucet JC, Yazdanpanah Y, Timsit JF. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. 2020 Apr;46(4):579-582. doi:10.1007/s00134-020-05967-x
- Sztrymf B, Messika J, Bertrand F, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med. 2011 Nov;37(11):1780-1786. doi:10.1007/s00134-011-2354-6
- Roca O, Caralt B, Messika J, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019 Jun 1;199(11):1368-1376. doi:10.1164/rccm.201803-0589OC
- Zhou X, Liu J, Pan J, Xu Z, Xu J. The ROX index as a predictor of high-flow nasal cannula outcome in pneumonia patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis. BMC Pulm Med. 2022 Apr 1;22(1):121. doi: 10.1186/s12890-022-01914-2
- Fielding-Singh V, Matthay MA, Calfee CS. Beyond low tidal volume ventilation: treatment adjuncts for severe respiratory failure in acute respiratory distress syndrome. Crit Care Med. 2018 Nov;46(11):1820-1831. doi:10.1097/CCM.0000000000003406
- Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med. 2020 May;8(5):433-434. doi:10.1016/S2213-2600(20)30127-2
