ACEP ID:
- My Account
- My CME
- Sign Out
ACEP ID:
Author: ACEP Emergency Ultrasound Section
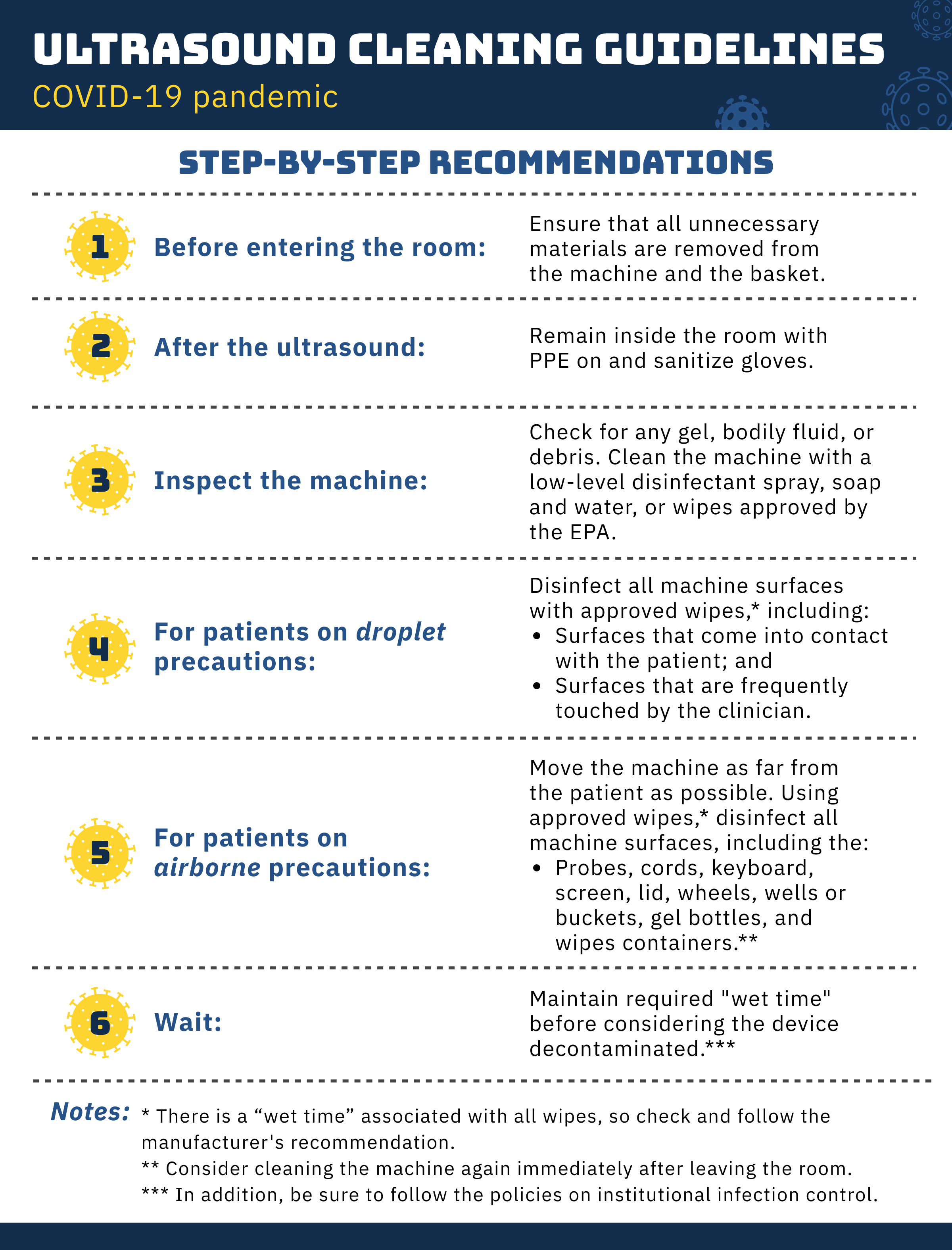
The ACEP Emergency Ultrasound Section wishes to provide guidance for cleaning and disinfection of ultrasound equipment in the context of the COVID-19 pandemic (Figure 9.4). Special guidance regarding COVID-19 includes the following:
This prevents unnecessary items from contamination by droplets and may include removal of nonessential transducers or extraneous items (eg, peripheral IV cannulas, plastic film dressing, bags holding towels).
We recommend that, before cleaning, clinicians remove gel and debris, and then use one of the EPA recommended products in between each patient encounter to disinfect the probe.1 Clinicians may find it advantageous to use a double-glove technique to help avoid cross-contamination from bare hands during the cleaning process.
Due to the recent knowledge that SARS-CoV-2, the causative agent of COVID-19, can be present on surfaces for days, we recommend disinfecting surfaces that either come into contact with the patient (eg, cable, transducer) or are touched by the clinician (eg, keyboard, screen, handlebar).2 We recommend that clinicians remove gel and debris, and then use one of the EPA recommended products in between each patient encounter.1,3
We recognize that many clinicians will not have access to transparent covers for ultrasound systems. In such cases, the entire ultrasound system and frequently touched surfaces should be disinfected with LLD solution between each patient.4
When performing an ultrasound examination in critically ill patients requiring active resuscitation where aerosolization is a risk (eg, intubation, medication nebulization, chest compressions, noninvasive ventilation), the machine and its components should be protected as much as possible.1,5 This includes use of probe covers (sterile and nonsterile) and may involve draping material, such as translucent bags. These covers should be discarded prior to exiting the patient’s room, taking care to avoid cross-contamination, in keeping with local infection control recommendations.
Refer to the current ACEP “Guideline for Ultrasound Transducer Cleaning and Disinfection” to determine when to use HLD.3 There is no evidence that HLD is beneficial for disinfection from SARS-CoV-2.
For ultrasound use during procedures such as peripheral or central venous access, a sterile probe cover should be used, followed by LLD in accordance with the ACEP “Guideline for Ultrasound Transducer Cleaning and Disinfection.”
The stocking of different solutions and products varies across the country, and some systems are facing shortages of certain products. We recommend that, in conjunction with infection control, physicians and health systems consider common disinfectants for cleaning, if there are no alternatives to commercial health care products. Examples include soap and water, diluted bleach, and ammonium chloride derivatives. This should be discussed with the vendor to prevent inadvertent destruction of machine elements.
Figure 9.4 Cleaning and disinfection of ultrasound equipment during the COVID-19 pandemic.