
Angiotensin II: A new tool in the management of refractory antihypertensive overdose
Case
A 50-year-old man with a history of hypertension, hyperlipidemia, borderline diabetes and depression was brought into the local Emergency Department (ED) after a reported overdose of his antihypertensive agents. His wife found him unresponsive on the ground with a suicide note. He was surrounded by empty bottles of his antihypertensive medications: metoprolol, amlodipine, and enalapril. The bottles indicated that they were last refilled 15 days ago for a 30-day supply. Upon arrival he was bradycardic, hypotensive, and unresponsive. His initial vital signs were Temp 98 °F, Pulse 45, BP 81/45 (MAP 57 mmHg), RR 16, and SpO2 98% on RA. His exam was significant for bradycardia and warm extremities with bounding pulses. He was intubated with only ketamine and resuscitation was initiated with a 1-liter normal saline bolus and infusions of both norepinephrine, at 20 mcg/min, and epinephrine, at 10 mcg/min. Hyperinsulinemia euglycemia (HIE) was also started early at 1 unit/kg/hr.
After 1 hour, he was still in the Emergency Department and despite adequate volume resuscitation and escalating the norepinephrine and epinephrine infusions, to 50 mcg/min each, and the HIE to 3 units/kg/hr, he remained bradycardic and his MAP was still below a goal of 65 mmHg.
Emergency Department Work-up:
- CBC: WBC 12.0, Hgb 13.0, HCT 39.9, MCV 102, Platelets 188
- Chemistry: Na 139, K 4.0, Cl 101, CO2 28, BUN 25, Cr 0.9, Glu 400. Anion Gap 10
- Acetaminophen <5, Salicylate: 1, Urine Drug Screen negative for amphetamines, cocaine, opiates, fentanyl, methadone, and THC.
- Head CT: no acute hemorrhage or mass.
- EKG: Sinus Bradycardia at 45 bpm with a PR of 202 ms, QRS 98 ms and QTc 450 ms.
Question
What additional agent(s) can be considered in this patient? What has been shown to be efficacious for treatment of distributive shock?
Background
Overdoses due to antihypertensive agents are common, with 108,614 exposures, and 326 deaths (170 from calcium channel blockers, 118 due to beta-blockers, and 38 from ACEi’s), reported to the National Poison Data System in 2017 (1). Patients can be profoundly toxic with agents that cause cardiotoxicity, vasodilation, or both (2). Multiple agent ingestions are even more difficult to manage due to synergy between different families of medications. There is much information published on beta-blocker and calcium channel overdoses. High dose vasopressors, and HIE have both been reported to successfully reverse hypotension due to antihypertensive overdoses (3), but some patients do not respond to even the highest doses of these treatments.
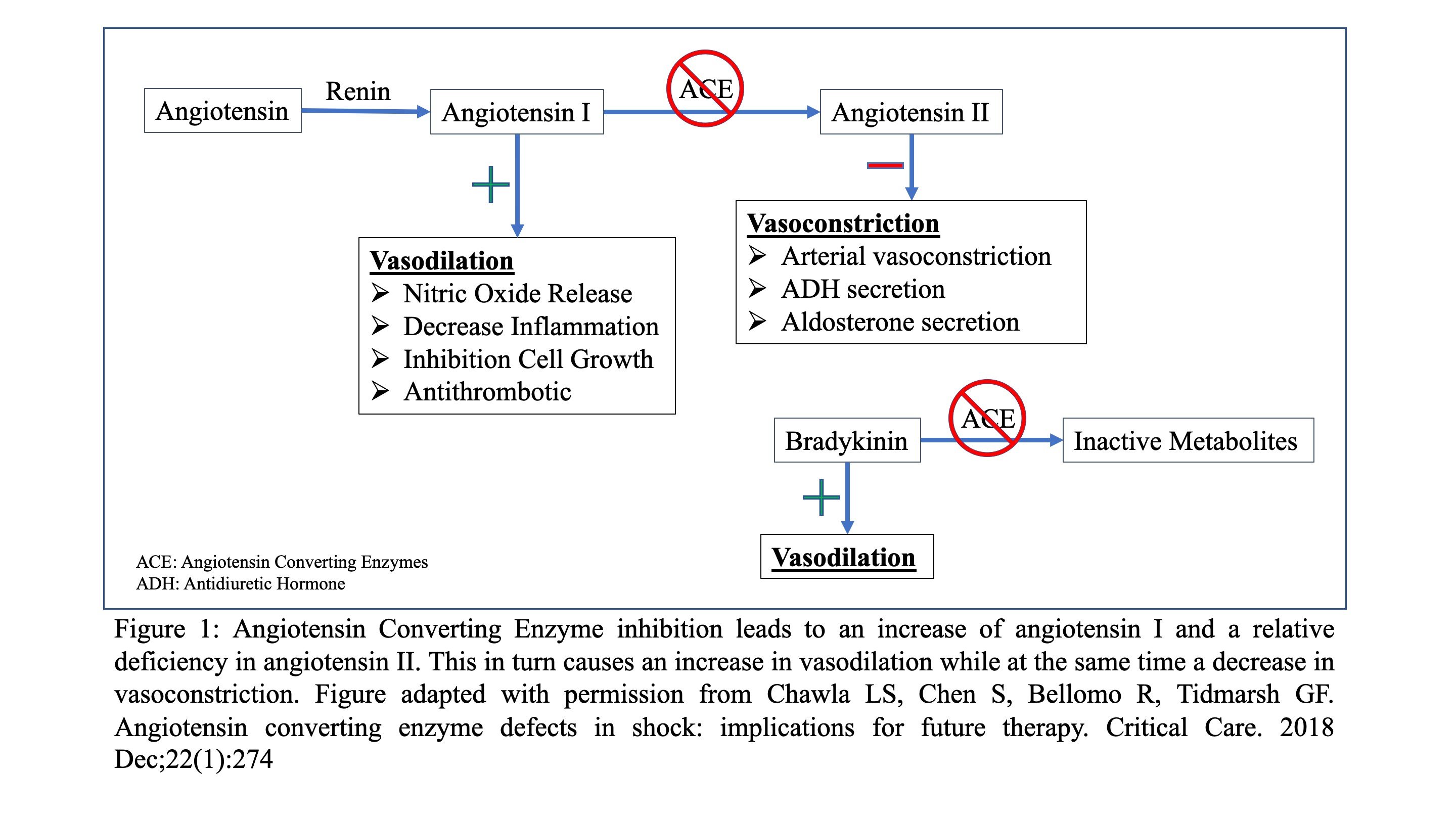
Angiotensin converting enzyme inhibitors (ACEi) are agents that work on the Renin-Aldosterone Angiotensin System (RAAS) (4). (Figure 1) The most well-known mechanism by which they decrease blood pressure is by blocking the conversion of angiotensin I to angiotensin II (ANGII), a molecule which causes vasoconstriction as well as ADH and aldosterone section (4). Additionally, decreased conversion of angiotensin I to ANGII leads to an excess of angiotensin I, which in turn causes an increase in nitric oxide release and, ultimately, vasodilation (5). Lastly, ACEi’s prevent the breakdown of bradykinin which also results in vasodilation (6-8).
Overdoses of ACEi are typically mild and well tolerated (9, 10), however, there are reports of significant catecholamine resistant hypotension (11-13). It is believed that patients who are chronically on ACEi’s may be at increased risk for refractory hypotension, due to a deficiency in ANGII (8). Therefore, ANGII may be a valuable and efficacious adjunctive medication in refractory hypotension due an antihypertensive overdose, especially in people who are on, or have overdosed with, an ACEi (14, 15).
Human ANGII is FDA approved for all causes of distributive shock. Although the stage III clinical trial that led to its approval (ATHOS-3) primarily enrolled patients suffering from septic shock (16), ANGII has previously been used to successfully treat hypotension from ACEi overdoses (11-13). The exact mechanism by which ANGII increases blood pressure is yet to be elucidated, but it is likely related to vasoconstriction and improved catecholamine release (17), through the binding of ANGII to a G-protein-coupled ANGII type 1 receptor on vascular smooth muscle cells, causing smooth muscle contraction via stimulation of Ca(2+)/calmodulin-dependent phosphorylation of myosin (18).
ANGII is administered as an infusion, with a starting rate of 20 ng/kg/min. It can be titrated every 5 minutes at increments up to 15 ng/kg/min. The half-life of ANGII is only 1 minute, allowing for rapid titration and discontinuation. The maximum rate in the first three hours of treatment is 80 ng/kg/min, and the maximum maintenance dose is 40 ng/kg/min. After the goal blood pressure has been achieved, other vasopressors can be weaned off. When the patient begins to clinically improve, ANGII can be down titrated every 5 to 15 minutes in increments as high as 15 ng/kg/min (18).
There are no known contraindications to the use of ANGII, although it has not been evaluated in pediatric patients. The most significant adverse event associated with its use is the risk of developing an arterial or venous thrombosis or thromboembolism, most commonly a deep vein thrombosis. In the ATHOS-3 trial, 13% of patients administered ANGII developed a thrombus or thromboembolism compared to 5% of the patients in the control arm (16). Therefore, thromboembolism prophylaxis should be considered in patients receiving ANGII.
Case Conclusion
The patient was started on ANGII at a dose of 20 ng/kg/min. Within 10 minutes, the blood pressure normalized with a mean arterial pressure of 65 mmHg. The norepinephrine and epinephrine were weaned off over the next hour, and the HIE was discontinued 2 hours later. The patient was maintained on ANGII in the ICU for the next day, at which time it too was also weaned off. The patient was successfully discharged to inpatient psychiatry without any sequelae.
References:
- Gummin DD, Mowry JB, Spyker DA, Brooks DE, Osterthaler KM, Banner W. 2017 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018:1-203.
- Hessler R. Cardiologic Principles II: Hemodynamics. In: Hoffman R, Howland M, Lewin N, Nelson L, Goldfrank L, editors. Goldfrank’s Toxicologic Emergencies. Tenth Edition ed. New York: McGraw-Hill; 2015. p. 222-30.
- St-Onge M, Anseeuw K, Cantrell FL, Gilchrist IC, Hantson P, Bailey B, et al. Experts consensus recommendations for the management of calcium channel blocker poisoning in adults. Crit Care Med. 2017;45(3):e306.
- Luque M, Martin P, Martell N, Fernandez C, Brosnihan KB, Ferrario CM. Effects of captopril related to increased levels of prostacyclin and angiotensin-(1-7) in essential hypertension. Journal of hypertension. 1996;14(6):799-805.
- Zhang W, Chen X, Huang L, Lu N, Zhou L, Wu G, et al. Severe sepsis: low expression of the renin-angiotensin system is associated with poor prognosis. Experimental and therapeutic medicine. 2014;7(5):1342-8.
- Chappell MC, Diz DI, Yunis C, Ferrario CM. Differential actions of angiotensin-(1-7) in the kidney. Kidney International. 1998;54:S3-S6.
- Margolius HS. Kallikreins and kinins: molecular characteristics and cellular and tissue responses. Diabetes. 1996;45(Supplement 1):S14-S9.
- Chawla LS, Chen S, Bellomo R, Tidmarsh GF. Angiotensin Converting Enzyme Defects in Shock: Implications for Future Therapy. Critical Care. 2018;In Press.
- Varughese A, Taylor AA, Nelson EB. Consequences of angiotensin-converting enzyme inhibitor overdose. American Journal of Hypertension. 1989;2(5_Pt_1):355-7.
- Lip G, Ferner R. Poisoning with anti-hypertensive drugs: angiotensin converting enzyme inhibitors. J Hum Hypertens. 1995;9(9):711-5.
- Jackson T, Corke C, Agar J. Enalapril overdose treated with angiotensin infusion. The Lancet. 1993;341(8846):703.
- Trilli LE, Johnson KA. Lisinopril overdose and management with intravenous angiotensin II. Ann Pharmacother. 1994;28(10):1165-8.
- Newby D, Lee M, Gray A, Boon N. Enalapril overdose and the corrective effect of intravenous angiotensin II. Br J Clin Pharmacol. 1995;40(1):103-4.
- Azizi M, Chatellier G, Guyene T-T, Murieta-Geoffroy D, Ménard J. Additive effects of combined angiotensin-converting enzyme inhibition and angiotensin II antagonism on blood pressure and renin release in sodium-depleted normotensives. Circulation. 1995;92(4):825-34.
- Bussard RL, Busse LW. Angiotensin ii: a new therapeutic option for vasodilatory shock. Therapeutics and Clinical Risk Management. 2018;14:1287.
- Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, et al. Angiotensin II for the Treatment of Vasodilatory Shock. N Engl J Med. 2017;377(5):419-30.
- Hall A, Busse LW, Ostermann M. Angiotensin in Critical Care. Critical Care. 2018;22(1):69.
- Product Information: Giapreza(TM) intravenous injection, angiotensin II intravenous injection. La Jolla Pharmaceutical Company (per manufacturer), San Diego, CA, 2017.
Brian Patrick Murray, DO and Joseph E. Carpenter, MD
Senior Medical Toxicology Fellows
Georgia Poison Center and Emory University School of Medicine, Atlanta, GA



