Emerging Ultrasound Technology: Sonothrombolysis in the treatment of acute ST-elevation MI
Tobias Kummer, MD, FACEP
Daniel Fiterman Molinari, MD
Larissa T. Shiue, MD
Department of Emergency Medicine, Mayo Clinic, Rochester
Introduction
Emergent percutaneous interventions (PCI) have revolutionized the care for patients with acute occlusive myocardial infarction and have been the treatment standard for decades. While PCIs have significantly improved the prognosis of patients with acute ST-segment elevation myocardial infarction (STEMI), significant microvascular obstruction (MVO) can still be shown in over half the patients undergoing emergent intervention, leading to persistent cardiac dysfunction.1 Sonothrombolysis using an ultrasound contrast agent (UCA) may benefit these patients by dissolving microvascular thrombi and improving coronary artery flow by nitric oxide (NO) release.2
The Science Behind Sonothrombolysis
At the core of sonothrombolysis lies the interaction between ultrasound pulses and the UCA. UCAs are microbubbles with an inert gas core and are approximately the size of an erythrocyte. When imaged with low acoustic energy (low mechanical Index - MI), the bubbles oscillate continuously and can be used to image blood flow. When insonated with a high energy pulse (high MI), the bubbles violently expand and abruptly collapse, causing a destructive microcavitation shock wave capable of dissolving microvascular thrombi. The shear force also induces endothelial and erythrocyte nitric oxide release, leading to local vasodilation and improved blood flow.3
How-to: the Sonothrombolysis protocol
A commercial ultrasound system with contrast-specific imaging software is used. Most published studies have used commercially available perflutren microbubbles (Definity, Lantheus Medical Imaging, North Billerica, MA.)2,4,5 This is diluted in 50mL normal saline and run as a continuous infusion at 1 -5 mL/min optimized to visualize myocardial perfusion while avoiding shadow artifacts in the far fields from too high contrast concentration.2,6 The perfusion of the myocardium is visualized using low (<0.3) MI imaging. Then, in repeating intervals, a series of short, high MI (>1.5) pulses are sent to destroy the bubbles. This is done repeatedly over all three apical cardiac windows (apical 2-, 3-, and 4-chamber).
Sonothrombolysis in acute STEMI has been studied pre- and post-PCI. A small case series of pre-hospital sonothrombolysis has demonstrated feasibility but larger studies are needed to evaluate safety and efficacy.4,6
Preliminary Data: Decreased infarct size and preserved systolic function
The Microvascular Recovery with Ultrasound in Acute Myocardial Infarction (MRUSMI) Trial is the largest trial to date and compared 50 patients that underwent primary PCI with pre-and post-PCI sonothrombolysis (total average time 50 min) to a control group of 50 patients that was treated with PCI only.2 This trial showed significantly higher resolution of ST-segment elevation prior to PCI (32% vs. 4%) and increased angiographic recanalization rates (48% vs. 20%). While the pre-PCI Left Ventricular Ejection Fraction (LVEF) was not different (44% vs 43%), it increased after PCI in the treatment group (47% vs. 43%) with a sustained difference at 6 months follow up (53% vs 47%). The need for implantable defibrillation (LVEF ≤ 30%) was reduced in the treatment group (5% vs. 18%).
A 2020 subanalysis of the MRUSMI trial showed decreased infarct size and more favorable myocardial strain,7 while a 2023 subanalysis showed improved diastolic function and left atrial mechanics.8
Side-effects and theoretical harms of Sonothrombolysis
All UCAs can cause potential anaphylactoid reactions, which are thought to be rare and less than with contrast used in Computed Tomography (CT).9 However, recent data suggest that UCAs containing polyethylene glycol might have a higher rate of severe adverse advent than previously reported.9,10 In animal models with high UCA doses and acoustic amplitudes, cavitation of microbubbles is potentially arrhythmogenic.11 However, this has not been observed in human trials under diagnostic imaging settings. However, the Reduction of Microvascular Injury Using Sonolysis (ROMIUS) Trial was halted early after three out of the first six patients had an unexpectedly high incidence of coronary vasoconstriction.12 The ROMIUS trial used extra-long pulse durations, which were later shown to cause vasoconstriction in animal studies.
Conclusion and Future Directions
While the MRUSMI trial was promising, no large-scale multicenter trials are currently available to validate these results externally. A planned pre-hospital trial in the Netherlands holds the promise of using the EMS transport time for a therapeutic intervention.6 Given the current technology, equipment, and operator training requirements, this currently will only be feasible in select high-resource settings.
However, sonothrombolysis shows promise in improving long-term outcomes for patients with acute occlusive myocardial infarction, which could also impact care for patients with no or limited access to primary PCI. Should the results of these early trials be replicated, it could be one of the most meaningful advancements in the care of patients with acute coronary occlusion since the introduction of thrombolytic therapy and PCI.
 Used with permission of Dr. Wilson Mathias
Used with permission of Dr. Wilson Mathias
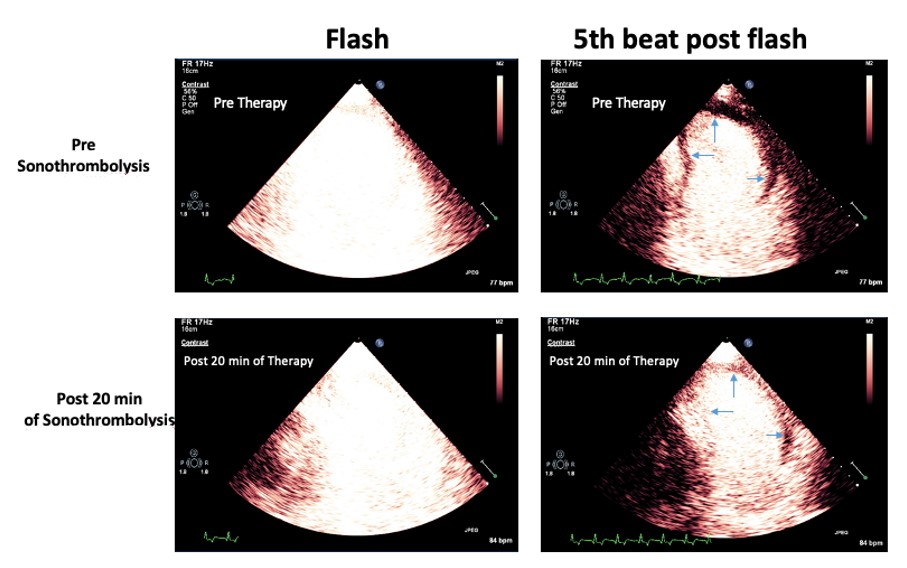
Figure. An acute anterior STEMI with a pain to door time of 45 minutes treated with sonothrombolysis using intermittent high MI impulses every 20 seconds during an intravenous infusion of lipid-encapsulated perfluorocarbon microbubbles (DefinityÒ). Top Panel: Low MI images were recorded to examine the risk area (blue area pointed by the arrows). Lower Panel: After 20 min of high MI impulses application during intravenous infusion of DefinityÒ, complete refilling of myocardial contrast is observed in medial and apical segments of anterior, septal and lateral walls, showing microvascular perfusion and predictable recovery of left ventricular function at follow-up. MI = mechanical index.
References
- Aggarwal S, Xie F, High R, Pavlides G, Porter TR. Prevalence and Predictive Value of Microvascular Flow Abnormalities after Successful Contemporary Percutaneous Coronary Intervention in Acute ST-Segment Elevation Myocardial Infarction. J Am Soc Echocardiogr. 2018;31:674-82.
- Mathias W Jr, Tsutsui JM, Tavares BG, Fava AM, Aguiar MOD, Borges BC, et al. Sonothrombolysis in ST-Segment Elevation Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention. J Am Coll Cardiol. 2019;73:2832-42.
- Xie F, Gao S, Wu J, Lof J, Radio S, Vignon F, et al. Diagnostic ultrasound induced inertial cavitation to non-invasively restore coronary and microvascular flow in acute myocardial infarction. PLoS One. 2013;8:e69780.
- El Kadi S, Porter TR, Zanstra M, Siegers A, van Loon RB, Hopman LHGA, et al. Feasibility of sonothrombolysis in the ambulance for ST-elevation myocardial infarction. Int J Cardiovasc Imaging. 2022;38:1089-98. https://doi.org/10.1007/s10554-021-02487-7.
- Bainey KR, Abulhamayel A, Aziz A, Becher H. Sonothrombolysis Augments Reperfusion in ST-Elevation Myocardial Infarction With Primary Percutaneous Coronary Intervention: Insights From the SONOSTEMI Study. CJC Open. 2022;4:644-6.
- El Kadi S, Porter TR, van Rossum AC, Kamp O. Sonothrombolysis in the ambulance for ST-elevation myocardial infarction: rationale and protocol. Neth Heart J. 2021;29:330-7.
- Aguiar MOD, Tavares BG, Tsutsui JM, Fava AM, Borges BC, Oliveira MT Jr, et al. Sonothrombolysis Improves Myocardial Dynamics and Microvascular Obstruction Preventing Left Ventricular Remodeling in Patients With ST Elevation Myocardial Infarction. Circ Cardiovasc Imaging. 2020;13:e009536.
- Chiang HP, Aguiar MOD, Tavares BG, Rosa VEE, Gomes SB, Oliveira MT Jr, et al. The Impact of Sonothrombolysis on Left Ventricular Diastolic Function and Left Atrial Mechanics Preventing Left Atrial Remodeling in Patients With ST Elevation Acute Myocardial Infarction. J Am Soc Echocardiogr. 2023;36:504-13.
- Shang Y, Xie X, Luo Y, Nie F, Luo Y, Jing X, et al. Safety findings after intravenous administration of sulfur hexafluoride microbubbles to 463,434 examinations at 24 centers. Eur Radiol. 2022. https://doi.org/10.1007/s00330-022-09108-4.
- Ali MT, Johnson M, Irwin T, Henry S, Sugeng L, Kansal S, et al. Incidence of Severe Adverse Drug Reactions to Ultrasound Enhancement Agents in a Contemporary Echocardiography Practice. J Am Soc Echocardiogr. 2023. https://doi.org/10.1016/j.echo.2023.10.010.
- Rota C, Raeman CH, Child SZ, Dalecki D. Detection of acoustic cavitation in the heart with microbubble contrast agents in vivo: a mechanism for ultrasound-induced arrhythmias. J Acoust Soc Am. 2006;120:295-64.
- Roos ST, Juffermans LJM, van Royen N, van Rossum AC, Xie F, Appelman Y, et al. Unexpected High Incidence of Coronary Vasoconstriction in the Reduction of Microvascular Injury Using Sonolysis (ROMIUS) Trial. Ultrasound Med Biol. 2016;42:1919-28.