August 18, 2020
Deep Vein Thrombosis (DVT)
Jesse M. Schafer, MD and Sean Stickles, MD
I. Introduction and Indications
- Deep vein thrombosis (DVT) is a condition of thrombus formation within the deep peripheral veins. DVTs have the potential to propagate and become pulmonary emboli, which itself carries an estimated mortality rate between 10-30%.1
- Risk factors can include:1
- Genetic factors (family history, factor V Leiden, protein c deficiency, protein S deficiency)
- Acquired factors (age, cancer, obesity, antiphospholipid antibodies)
- Situational/Transient factors (pregnancy, oral contraceptives, immobilization, recent hospitalization/surgery/trauma)
- The use of point-of-care ultrasound (POCUS) for the evaluation of DVT has robust evidence, with estimates of sensitivity and specificity of 96.1% (95% CI 90.6-98.5%) and 96.8% (95% CI 94.6-98.1%), respectively.2
- POCUS has also been shown to hasten disposition in patients evaluated for DVT compared to comprehensive radiology ultrasound by as much as 2 hours.3
- Indications for the POCUS exam include:
- Leg swelling or pain
- Concern for pulmonary embolism and possible concomitant DVT
Anatomy
- Upper Extremity Venous Anatomy
- In the neck, around the level of the clavicle, the internal jugular meets the subclavian vein to form the brachiocephalic vein, which in turn flows into the superior vena cava.
- The subclavian vein (SCV) travels beneath the clavicle as the name implies. It is formed by the confluence of the cephalic vein and axillary vein.
- The axillary vein can be found in the proximal medial humeral region in the axilla.
- The axillary vein is formed by the confluence of the brachial vein and the basilic vein.
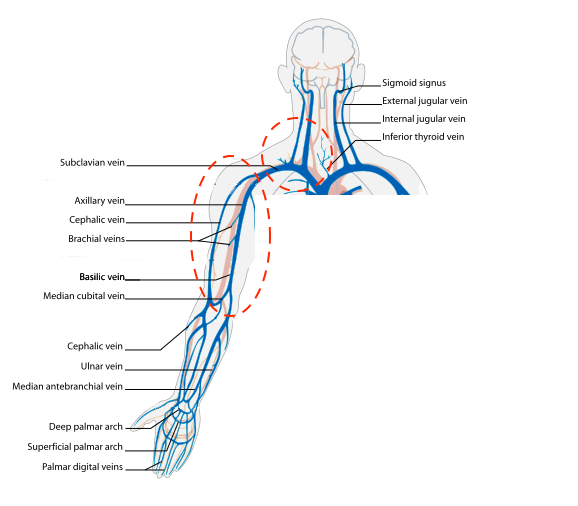
- Figure 1. Upper extremity venous anatomy (courtesy of Wikimedia)
- Lower Extremity Venous Anatomy
- The common femoral artery and vein emerge from beneath the inguinal ligament and traverse distally along the medial thigh. The inguinal crease is the surface landmark that corresponds to the inguinal ligament. The scanning protocol described below will begin in this region. ( 2, upper circle).
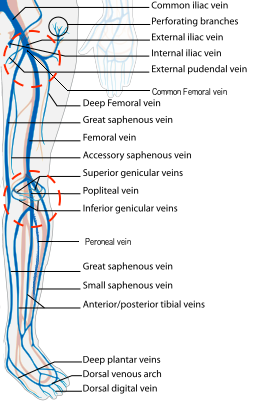
- Figure 2. Veins of the leg and zones of 2-region compression (courtesy of Wikimedia)
- The femoral artery and its branches are paired with the femoral vein and venous tributaries as they course through the thigh and leg (Video 1).
- The first venous confluence is the great saphenous vein (GSV) and common femoral vein (CFV). The GSV joins the CFV medially.
- The CFV forms a few centimeters distally to the GSV confluence, formed by the deep femoral vein (DFV) and femoral vein (FV). (The femoral vein is occasionally referred to as the superficial femoral vein, but as this structure is actually considered part of the deep venous circulation, it will be referred to as the femoral vein for the rest of this discussion.)
- The common femoral artery (CFA) splits at the same location (generally just proximal to the formation of the CFV) and forms the deep femoral artery and femoral artery (FA). From this point, the deep arteries and veins disappear from view, but the FA and FV can be imaged along the medial thigh until the adductor canal (AC), just proximal to the medial knee. The FA will be seen as the more superficial pulsatile structure with the FV just deep to the FA.
- Video 1. Scanning distally from GSV towards adductor canal
- After the FA and FV traverse the adductor canal to the posterior thigh and knee, they become the popliteal artery (PA) and popliteal vein (PV). The PV will be superficial to the PA.
- The PV will quickly trifurcate in the popliteal fossa. The visible tributaries in this region are the posterior tibial vein (PTV), anterior tibial vein (ATV), and peroneal or fibular vein ( 2, lower circle).
- The common femoral artery and vein emerge from beneath the inguinal ligament and traverse distally along the medial thigh. The inguinal crease is the surface landmark that corresponds to the inguinal ligament. The scanning protocol described below will begin in this region. ( 2, upper circle).
III. Scanning technique, normal findings and common variants
- Scanning technique
- Probe/transducer selection
- High frequency linear transducer (7mHz to 14mHz) with vascular setting
- Indicator to patient’s right
- Compression technique (Video 2)
- This exam involves compressing the identified veins and confluences.
- With the artery and vein in view, compress at a perpendicular angle with enough pressure to bring the walls of the vein together and slightly compress the walls of the artery.
- Make sure the vein is centered in the image to optimize compression.
- Video 2. Vein compression adequate enough to collapse walls of vein
- Upper Extremity Scanning Protocol
- Start in the neck to evaluate the internal jugular vein to the level of the brachiocephalic. With the patient’s head turned to the contralateral side, place the transducer in a transverse orientation over the sternocleidomastoid muscle. You should be able to identify the IJ over the carotid artery (CA) (Fig. 3). Compress if you do not see echogenic material in the lumen of the IJ.
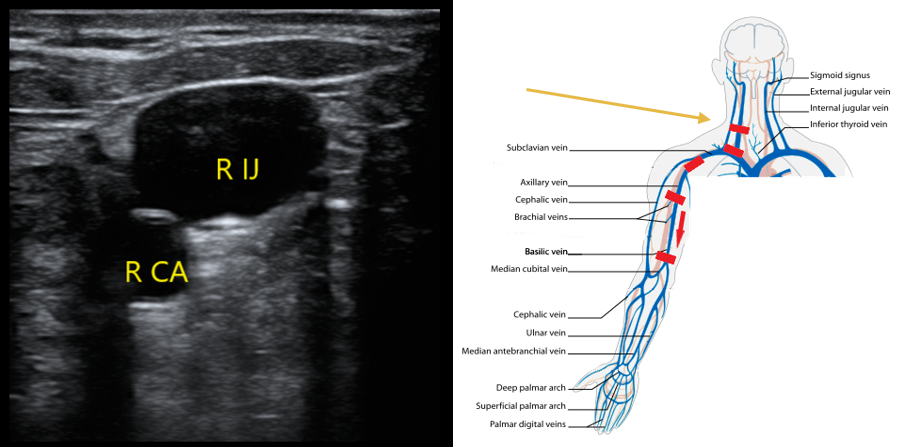
- Figure 3. Appearance of right internal jugular and right CA. Most caudal red rectangle indicates orientation of transducer.
- Follow the IJ to the confluence with the SCV where they form the brachiocephalic vein. Compression in this region will not be possible. Use color flow to evaluate for filling defects and pulsed wave Doppler to evaluate for flow patterns. Absent or monophasic patterns are abnormal. Biphasic or triphasic flow is normal.
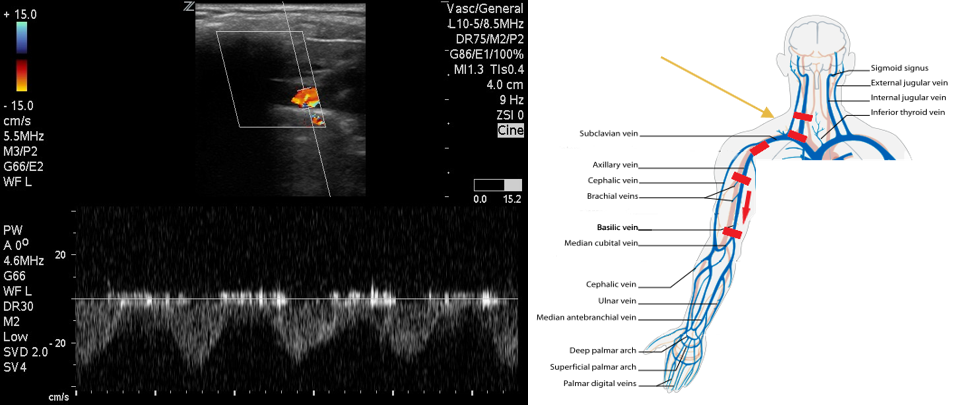
- Figure 4. Pulsed wave Doppler over subclavian vein demonstrates biphasic flow.
- Next move to the axilla on the same side. With the patient in supine position, have them place their hand on their head to expose the area of interest.
- Identify the subclavian vein as it crosses from the axilla. This area can be difficult to compress. Use color flow in long axis to identify filling defects. Use pulsed wave Doppler to identify normal versus abnormal flow patterns.
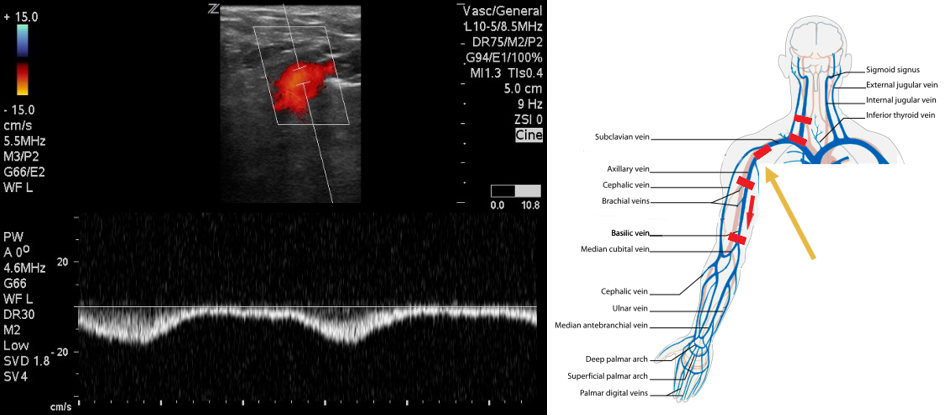
- Figure 5. Pulsed wave Doppler over axillary vein demonstrates biphasic flow.
- Follow the axillary vein as it traverses into the proximal arm and compress along the way (Fig. 6).
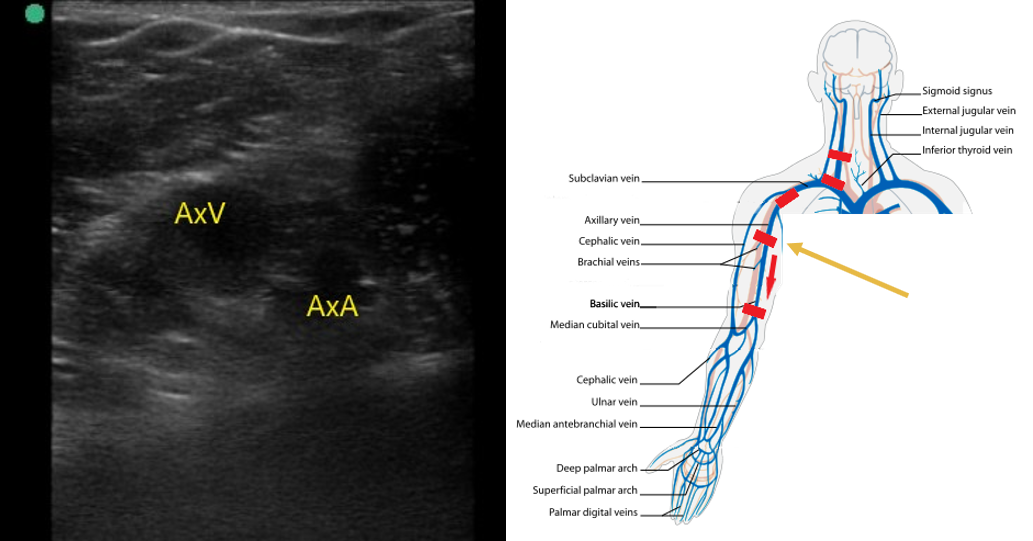
- Figure 6. Axillary artery (AxA) and axillary vein (AxV) in the proximal upper arm.
- The axillary vein going distally will split in the proximal humeral region into the basilic and 2 brachial veins (Fig. 7). The brachial veins will be paired with the brachial artery. The basilic vein is larger, more superficial, and runs medially between the biceps and triceps (Video 3).
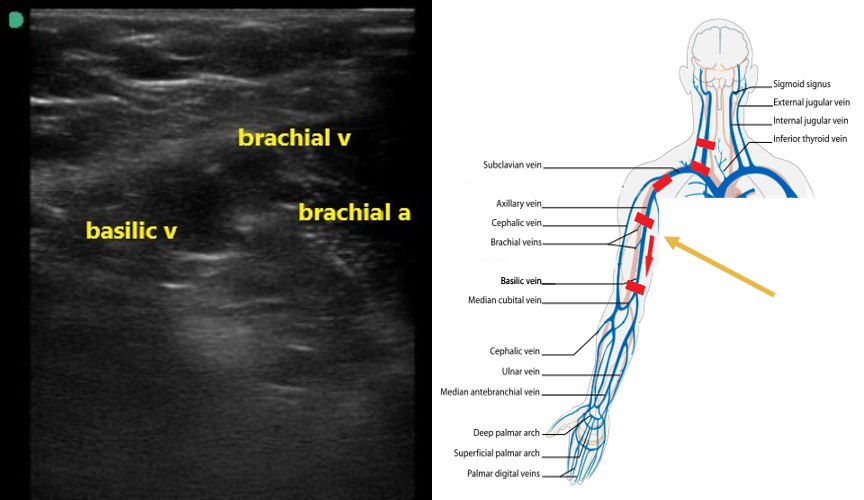
- Figure 7. Basilic and brachial veins as well as the brachial artery in the proximal upper arm.
- Video 3. Axillary vein scanning distally to split into basilic and 2 brachial veins.
- Follow these veins to the antecubital fossa, compressing along their course.
- Video 4. Compression of the veins in the upper arm.
- Start in the neck to evaluate the internal jugular vein to the level of the brachiocephalic. With the patient’s head turned to the contralateral side, place the transducer in a transverse orientation over the sternocleidomastoid muscle. You should be able to identify the IJ over the carotid artery (CA) (Fig. 3). Compress if you do not see echogenic material in the lumen of the IJ.
- Lower Extremity Scanning Protocol
- The point-of-care exam has typically been described as the 2-point or 2-region technique ( 2), although some centers utilize continual compression from the inguinal crease to popliteal fossa.
- Position the leg with the knee flexed in slight lateral rotation, in a “frog-leg” position.
- Identify the inguinal crease at the very proximal medial thigh and place the transducer in a transverse orientation as indicated in Fig. 8. You should see the CFA and CFV as in the corresponding ultrasound image. If you see three vessels (CFA, CFV, SV), you are not proximal enough. Compress the CFV (Fig. 8 and 9).
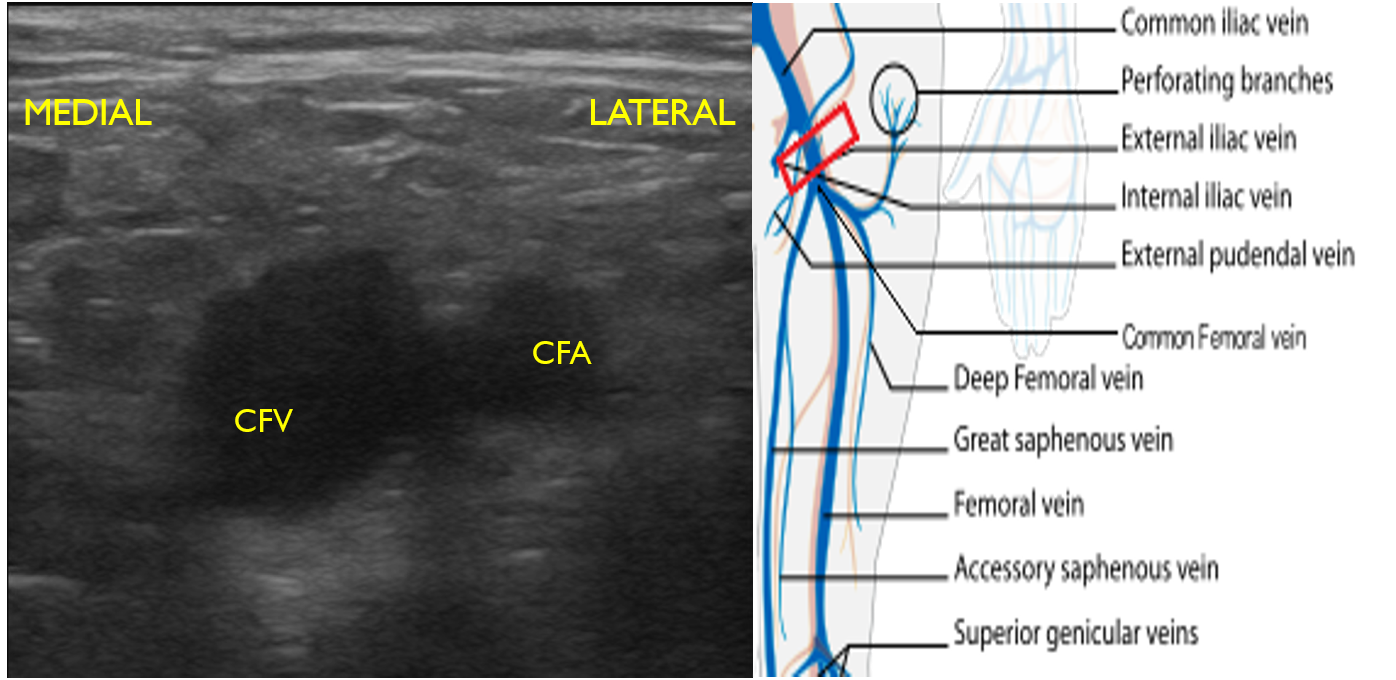
- Figure 8. Common femoral vein and artery at the inguinal crease with transducer orientation indicated (red rectangle)
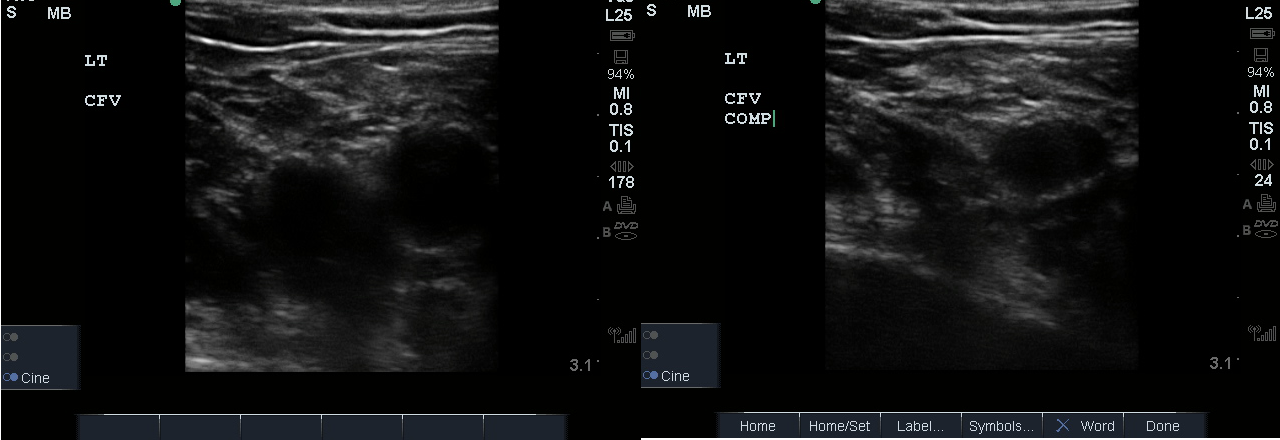
- Figure 9. Compression of the common femoral vein at the level of inguinal crease.
- Slide the transducer distally 1 to 2 cm to the junction of the CFV and GSV ( 10 and 11) and compress.
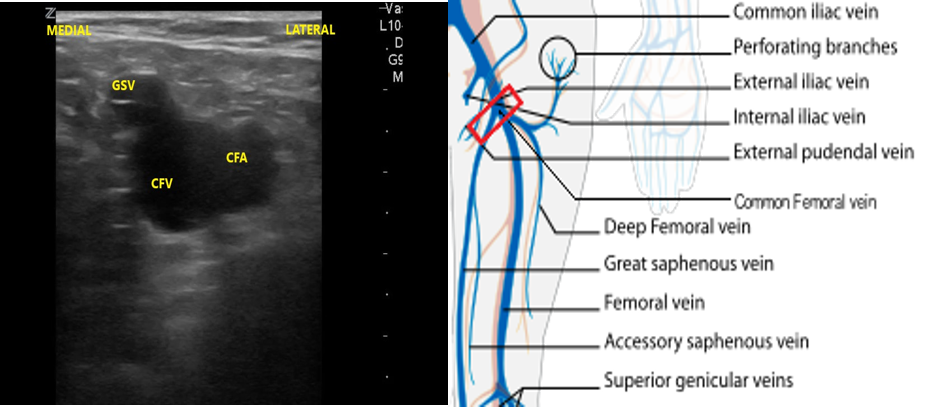
- Figure 10. Junction of the CFV and GSV 1-2 cm below the inguinal canal with transducer orientation indicated (red rectangle).
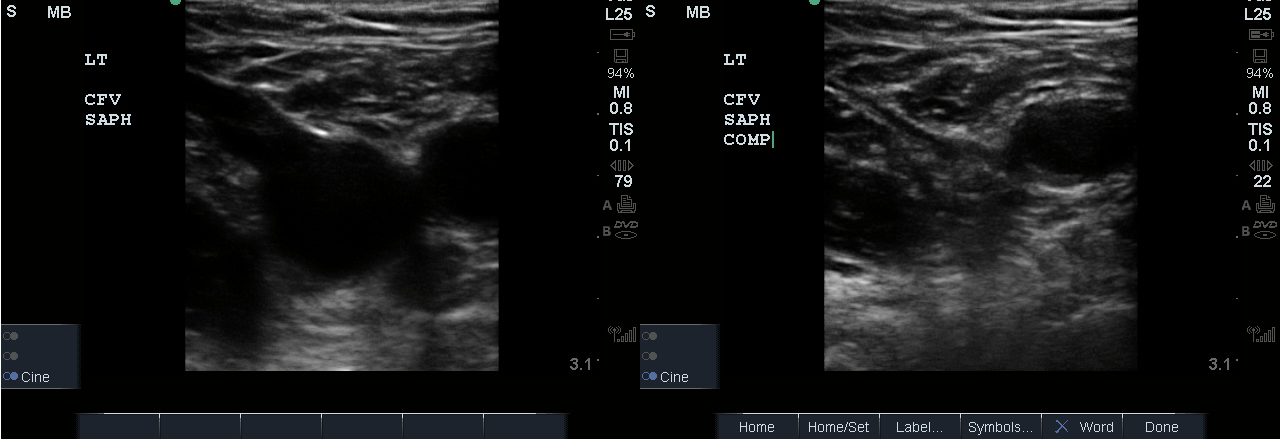
- Figure 11. Compression of the CFV at the junction of the GSV 1-2 cm below the inguinal crease.
- Slide the transducer another 1 to 2 cm (3-4 cm distal to the inguinal ligament) to the junction of the DFV and FV. You will also see the CFA branch into the FA and DFA (Fig. 12). This constitutes completion of evaluation of “region 1.”
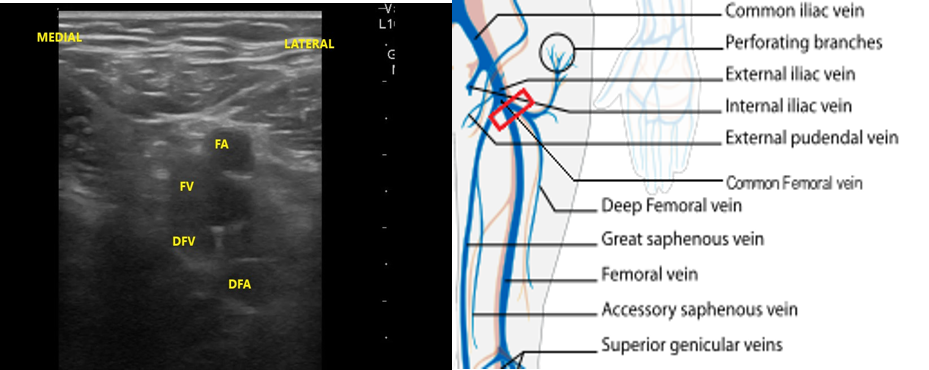
- Figure 12. Visualization of the DFV and FV just distal from where they converge into CF with transducer orientation indicated (red rectangle).
- For continual evaluation along the proximal leg, slide the transducer along the medial thigh, keeping the FA and FV in view. The FA will be superficial to the FV (Fig. 13). Compress at short intervals along the course of the vein until the pair dives deep through the adductor canal, just proximal to the knee.
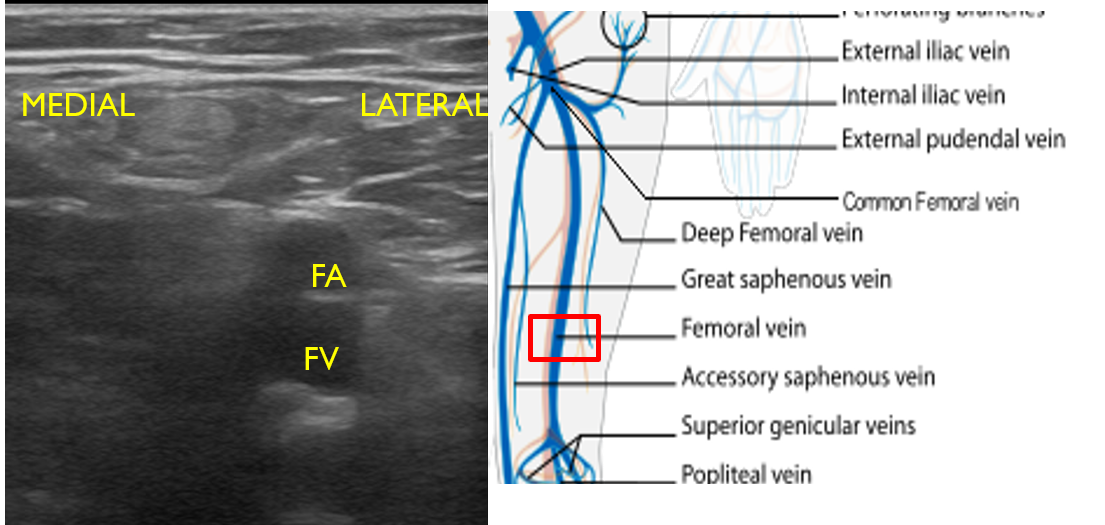
- Figure 13. Femoral artery and vein seen in mid to distal upper leg with transducer orientation indicated (red rectangle).
- Move the transducer to the proximal medial popliteal region with the knee slightly flexed.
- In the popliteal region, you should see the popliteal vein (PV) superficial to the popliteal artery (PA). (Fig. 14) If you see more than one artery and one vein, slide the transducer proximally until you see the PV and PA. When you have this view, compress as before.
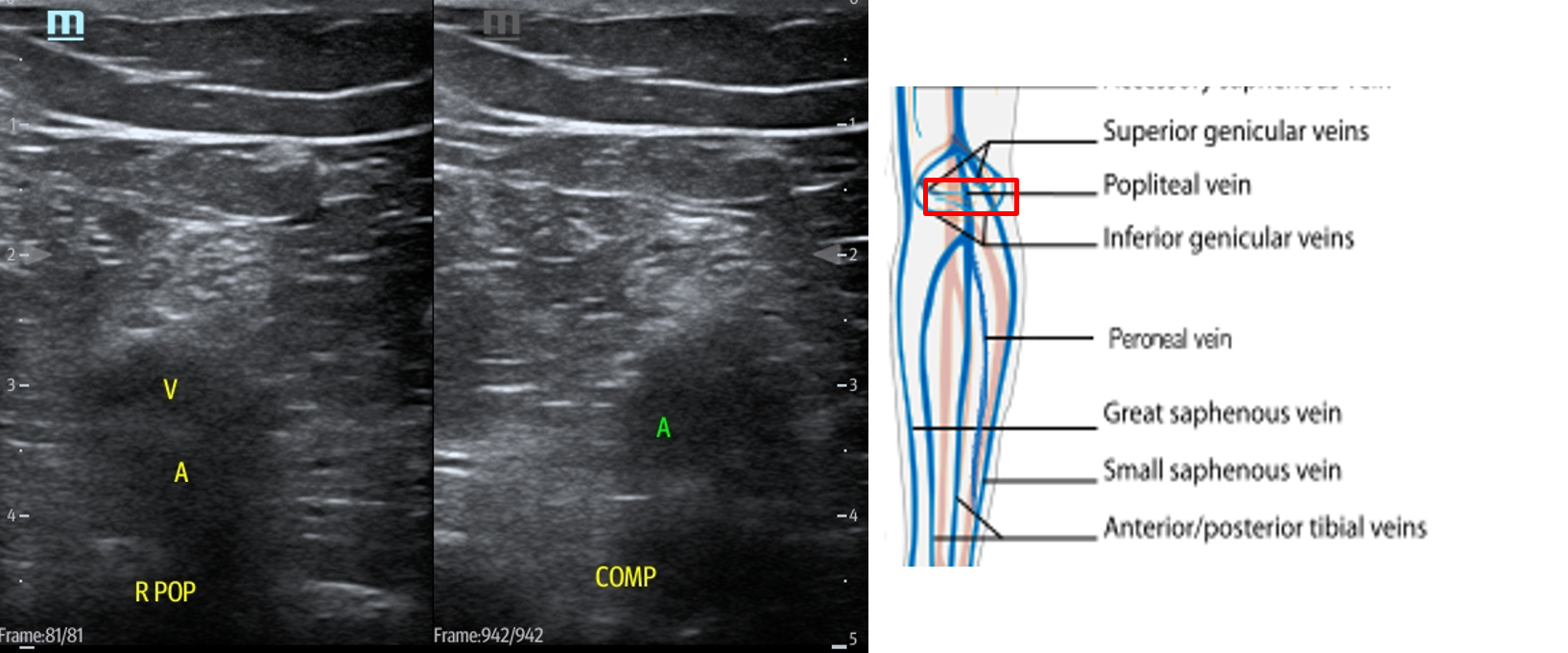
- Figure 14. Compression of the popliteal vein in the popliteal fossa with transducer orientation indicated (red rectangle).
- Slide the transducer distally until you see the PV trifurcate into the PTV, ATV and peroneal vein. (Fig. 15) Compress each of these veins. This constitutes completion of evaluation of “region 2.”
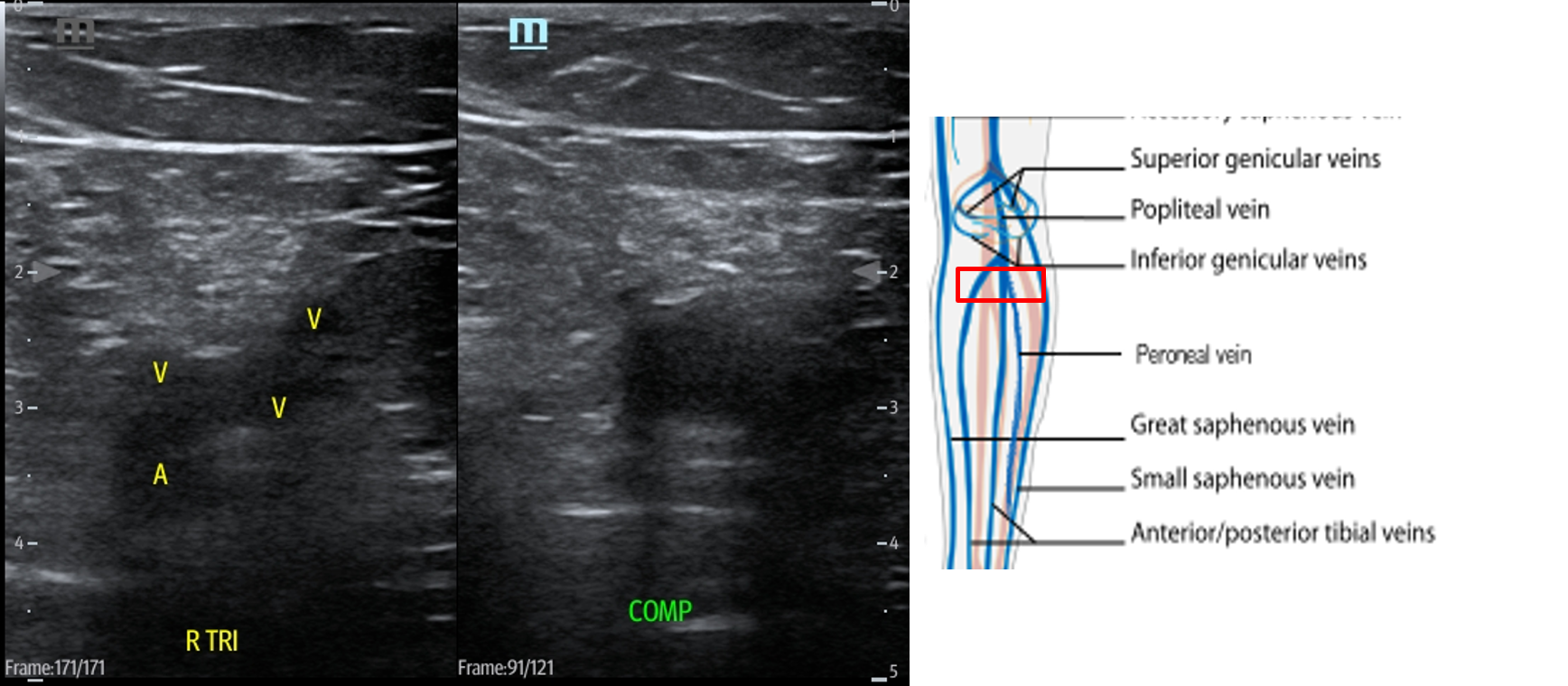
- Figure 15. Compression of the PTV, ATV and peroneal vein in the distal portion of the popliteal fossa just before they converge into the popliteal vein with transducer orientation indicated (red rectangle).
- Probe/transducer selection
- Adjuncts
- Augmentation
- Involves placing the transducer proximally on the leg, typically at the CFV, and setting the ultrasound machine to color or pulsed wave Doppler mode.
- The calf is then squeezed or compressed along the venous system in order to increase venous return.
- Video 5. Augmentation demonstrates color flow in proximal portion of vein when calf is squeezed.
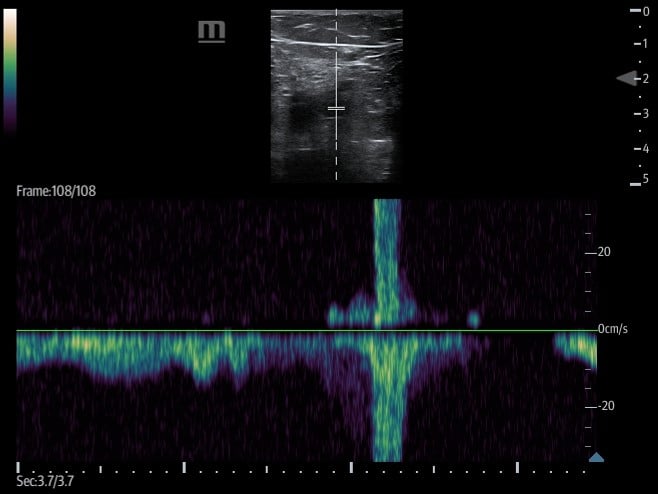
- Figure 16. Pulsed wave doppler demonstrating increased venous return with augmentation.
- If no DVT is present, there will be an increase in flow velocity noted on the ultrasound machine screen once the rush of blood passes the area where the transducer is placed.
- A lack of increased flow identified may be suggestive of an occlusive thrombus between the two points.
- Partial obstruction may can dampened flow when compared to the other side but can also cause subtle change in flow which can lead to false negative augmentation study.
- Respiratory variation/phasic flow
- Involves placing the transducer on the leg, typically at the CFV, and setting the ultrasound machine to spectral (pulsed wave) Doppler mode.
- Flow velocity in deep venous structures varies with the respiratory cycle and changes in intrathoracic pressure.
- A lack of variation in flow may be suggestive of an obstructive process (eg, thrombus, compressive pathology) along the venous system between the proximal inferior vena cava and point of transducer placement. (Figure 17)
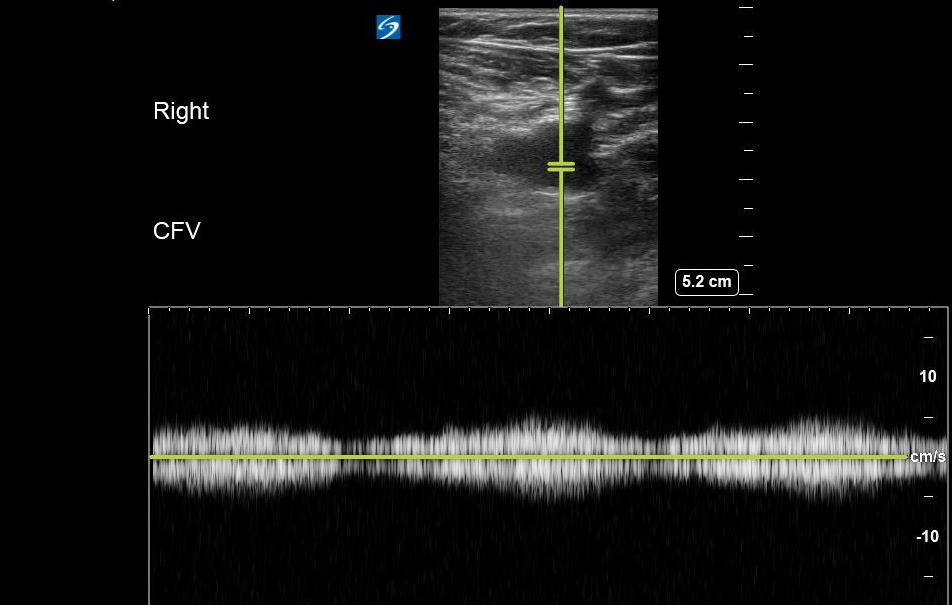
- Figure 17. Respiratory variation seen at the CFV with Pulsed wave Doppler.
- Normal findings
- Veins should be easily compressible and free of echogenic material.
- Anterior and posterior walls of the vein should touch with compression to confirm patency and absence of DVT. (Video 2)
- Occasionally, you may identify valves at or near the confluence of the veins. This is commonly seen at the SV and CFV junction. (Video 6)
- Video 6. Valve at junction of saphenous and common femoral veins
- Common variants
- In a retrospective study of 808 lower limb venograms, 42% of studies were noted to have two veins visualized in the popliteal fossa, and 31% of studies involved duplicated superficial femoral veins.4
- Augmentation
Pathology
- Significance of pathology
- Non-compressible veins are sign of DVT. Veins with acute DVT may not have internal echogenicity depending on acuity or chronicity
- Video 7. Acute DVT of the common femoral vein
- Video 8. Acute DVT of the popliteal vein
- Non-compressible veins are sign of DVT. Veins with acute DVT may not have internal echogenicity depending on acuity or chronicity
- Veins with echogenic material in the lumen can be seen with subacute or chronic DVT, and particularly in chronic DVT, are often adherent to the vessel wall.
- Incidence of Pathology
- Roughly 200,000 outpatients are diagnosed with DVT annually.5,6
- Incidence of DVT has been estimated at 7.1 per 10,000 annually.7,8
- Estimation of lower extremity DVT by location9
- Isolated popliteal vein- 14.6%
- Isolated femoral vein- 5.5%
- Isolated common femoral vein- 1.4%
- Isolated deep femoral vein- 0.8%
- The remainder were not isolated to one region
- Roughly 6% of patients with lower extremity DVT will have isolated DVT in a location other than the CFV or PV. The implication of this is that these cases would be missed by simple 2-point compression ultrasound as they in a location outside of those 2 areas.9
- Upper extremity DVT accounts for roughly 4% to 10% of all DVTs.10,11
- Significance of pathology (morbidity and mortality)
- Case fatality rate 9% of first isolated DVT in large cohort study.7
- Pulmonary embolus is estimated to occur in 6% of upper extremity DVT and 15 to 32% of lower extremity DVT. 7,12-15
Pearls and Pitfalls
- Tips on image acquisition and interpretation
- Adjust depth to allow clear visualization of pertinent vessels and minimize extraneous anatomy.
- Optimize gain settings to allow clear identification of pertinent vessels and differentiate from surrounding anatomy.
- Upper Extremity
- Upper extremity DVT can be difficult for the novice sonographer to perform and has not traditionally been incorporated in Emergency Medicine ultrasound training at the resident level. It is, however, part of the curriculum at the fellow level.16 Upper extremity DVT ultrasound by the novice sonographer should only be used to rule in DVT of the upper extremity. Where diagnostic uncertainty exists, further consultation or imaging evaluation must be considered.11,17
- DVTs are often identified by indirect methods such as color flow or spectral Doppler. If the wave pattern is abnormal--monophasic or absent-- compare with the unaffected side. If uncertainty exists, advanced imaging or specialty consultation should be considered.17
- Lower Extremity
- Systematic compression along the course of the vein rather than 2-point compression may increase sensitivity and specificity of this exam and decrease the false negative rate.9,18,19
- The sensitivity of 2-point compression ultrasound may be improved with the addition of d-dimer testing.20 Alternatively, patients may be advised to undergo follow-up testing in 7 days as outpatient to rule out for progression of distal or undetected DVT. However, one study noted that only 28% of high-risk patients who underwent initial 2-point compression ultrasound in the ED (and could be contacted after discharge) actually underwent their follow-up exam.21
- Patients who are morbidly obese may present a challenge when trying to evaluate for DVT using a linear transducer. In these cases, using a curvilinear transducer may allow better penetration through the soft tissues while maintaining adequate resolution to evaluate for possible DVT.22
- Common Mistakes
- Lymph nodes can appear as a rounded structure with internal echogenic material in the hium. These can be misinterpreted as a DVT.
- Video 9. Lymph node
- Superfical veins, if large, can be mistaken for the deep veins. Increase depth of view to identify appropriate deep veins.
- Baker’s cysts can be seen in the popliteal region and will be non- compressible. These are often much larger than nearby veins and will track toward the knee joint. One study indicated that almost 13% of patients getting DVT ultrasound had incidental findings, with Baker’s cyst being the most common (40%).23 (Video 10)
- Video 10. Baker’s cyst
- If you have inadequate compression or are compressing a vein at an angle other than perpendicular, the vein may not compress completely and can result in a false positive.
- Abscesses or hematomas can apear as rounded structures with echogenic material and potentially lead to false positive results.
References
- Beckman MG, Hooper WC, Critchley SE et al. Venous thromboembolism: a public health concern. Am J Preventive Med. 2010;38:S495-S501.
- Pomero F, Dentali F, Borretta V, et al. Accuracy of emergency physician-performed ultrasonography in the diagnosis of deep-vein thrombosis: a systematic review and meta-analysis. Thromb Haemost. 2013;109:137-45.
- Theodoro D, Blaivas M, Duggal S, et al. Real-time B-mode ultrasound in the ED saves time in the diagnosis of deep vein thrombosis (DVT). Am J Emerg Med. 2004;22:197-200.
- Quinlan DJ, Alikhan R, Gishen P, et al. Variations in lower limb venous anatomy: implications for US diagnosis of deep vein thrombosis. Radiology. 2003;228:443-8.
- Courtney DM, Kline JA. Identification of prearrest clinical factors associated with outpatient fatal pulmonary embolism. Acad Emerg Med. 2001;8:1136-42.
- Burnside PR, Brown MD, Kline JA. Systematic review of emergency physician-performed ultrasonography for lower-extremity deep vein thrombosis. Acad Emerg Med. 2008;15:493-8.
- Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
- Anderson FA Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933-8.
- Adhikari S, Zeger W, Thom C, et al. Isolated deep venous thrombosis: Implications for 2-point compression ultrasonography of the lower extremity. Ann Emerg Med. 2015;66:262-6.
- Isma N, Svensson PJ, Gottsater A, et al. Upper extremity deep venous thrombosis in the population-based Malmo thrombophilia study (MATS). Epidemiology, risk factors, recurrence risk, and mortality. Thromb Res. 2010;125:e335-8.
- Kucher N. Clinical Practice: Deep-vein thrombosis of the upper extremities. N Engl J Med. 2011; 364: 861-9.
- Owens CA, Bui JT, Knuttinen MG, et al. Pulmonary embolism from upper extremity deep vein thrombosis and the role of superior vena cava filters: a review of the literature. J Vasc Interv Radiol. 2010;21:779-87.
- Munoz FJ, Mismetti P, Poggio R, et al. Clinical outcome of patients with upper-extremity deep vein thrombosis: results from the RIETE Registry. Chest. 2008;133:143-8.
- Kucher N, Tapson VF, Goldhaber SZ. Risk factors associated with symptomatic pulmonary embolism in a large cohort of deep vein thrombosis patients. Thromb Haemost. 2005;93:494-8.
- Hughes MJ, Stein PD, Matta F. Silent pulmonary embolism in patients with distal deep venous thrombosis: systematic review. Thromb Res. 2014;134:1182-5.
- Lewiss RE, Tayal VS, Hoffmann B, et al. The core content of clinical ultrasonography fellowship training. Acad Emerg Med. 2014;21:456-61.
- Chin EE, Zimmerman PT, Grant EG. Sonographic evaluation of upper extremity deep venous thrombosis. J Ultrasound Med. 2005;24:829-38; quiz 39-40.
- Jang T, Docherty M, Aubin C, et al. Resident-performed compression ultrasonography for the detection of proximal deep vein thrombosis: fast and accurate. Acad Emerg Med. 2004;11:319-22.
- Zitek T, Baydoun J, Yepez S, et al. Mistakes and pitfalls associated with two-point compression ultrasound for deep vein thrombosis. West J Emerg Med. 2016;17:201-8.
- Bernardi E, Camporese G, Buller HR, et al. Serial 2-point ultrasonography plus D-dimer vs whole-leg color-coded Doppler ultrasonography for diagnosing suspected symptomatic deep vein thrombosis: a randomized controlled trial. JAMA. 2008;300:1653-9.
- McIlrath ST, Blaivas M, Lyon M. Patient follow-up after negative lower extremity bedside ultrasound for deep venous thrombosis in the ED. Am J Emerg Med. 2006;24:325-8.
- Zitek JA, Baydoun J, Baird J. Tools for the clinician: the essentials of bedside (ED or ICU) ultrasound for deep vein thrombosis. Curr Emerg Hosp Med Rep. 2013;1:65-70.
- Kocakoc E, Bhatt S, Dogra VS. Utility of venous compression in deep venous thrombosis evaluation revisited. Bratisl Lek Listy. 2012;113:417-20.