Use of Ultrasound in Cardiac Arrest
Eric Neasi MD, Emergency Medicine Senior Resident, University of Wisconsin-Madison
Hani I. Kuttab MD, Assistant Ultrasound Director, University of Wisconsin-Madison
Introduction
Point-of-care ultrasound (POCUS) is an invaluable tool in the setting of cardiac arrest as it can identify reversible causes of cardiac arrest. [1–3] and the presence or absence of cardiac activity. The presence of cardiac activity during pulse checks is associated with an increased likelihood of return of spontaneous circulation (ROSC) as well as survival, while the absence of cardiac activity is associated with an increased likelihood of mortality. [1,4–6] Most research has centered on transthoracic echocardiogram use (TTE). However, more emergency departments are now implementing point-of-care transesophageal echocardiogram (TEE) utilization in cardiac arrest.
What the Data Says
Pulse Checks Are Unreliable
Data has demonstrated that pulse checks are inaccurate and difficult to palpate due to a variety of reasons, such as patient habitus, varying techniques, or quality of pulse palpation amongst different users. One study showed that pulse palpation in a pediatric cohort was only 78% accurate. [7] Additionally, patients suffering from cardiac arrest are frequently in a state of profound shock, such that central pulses may be faint or weak, making them even more difficult to locate. In one study, 34 patients were identified to have no pulse, 11 of which actually had sonographic evidence of cardiac activity on ultrasound. [4] In these situations, POCUS may provide a more accurate representation of a patient’s cardiac activity. A direct comparison of cardiac ultrasound to Doppler ultrasonography and manual palpation suggested that cardiac ultrasound more rapidly and accurately detected pulse than either manual palpation and Doppler ultrasonography. [8]
Cardiac Standstill Is Predictive of Mortality
Lack of cardiac activity on ultrasound in the setting of arrest indicates poor survival. Studies have shown a mortality rate of 100% if cardiac standstill was identified during a pulse check. [4,5,9] The largest trial to date, the REASON trial, enrolled 793 patients in pulseless electrical activity (PEA) and asystole. In this study, 263 patients (33%) had cardiac activity on initial ultrasound, while 530 patients (67%) did not. ROSC was achieved in >50% of cases if cardiac activity was detected by ultrasound. Cardiac activity was associated with increased survival to hospital admission and increased survival to hospital discharge (3.8%). Lack of cardiac activity on ultrasound was associated with non-survival, although 0.6% of patients without cardiac activity on ultrasound still survived to hospital discharge. Thus, the absence of cardiac activity is not 100% sensitive for mortality. [1]
Pseudo-PEA
Pseudo-PEA is defined as sonographic cardiac activity but without a palpable pulse. [10] It is critical for emergency medicine practitioners to distinguish this from PEA as treatment strategies between the two differ. Additionally, prognosis between the two also differs: pseudo-PEA is associated with higher rates of ROSC and survival [10]. A secondary analysis of the REASON trial by Gaspari et al. divided those patients in PEA/asystole and with initial cardiac activity on ultrasound as ‘organized’ activity, defined as coordinated contractions of the myocardium resulting in a change of volume of the left ventricle, versus ‘disorganized’ activity, defined as ‘agonal twitching’. [11] Furthermore, the initiation of continuous adrenergic agents (e.g., an epinephrine infusion) in patients with organized cardiac activity also improved survival compared to those with disorganized activity, suggesting that POCUS may identify subsets of patients with PEA and asystole who may respond differently to resuscitation. [11]
Logistical Considerations of Performing Ultrasound during Cardiac Arrest
The phased array probe is the preferred probe for use during cardiac arrest as it optimizes the detection of motion and has a smaller footprint to better visualize the heart through the subxiphoid (SX) and parasternal long axis (PSL) windows. The SX view is a strategic choice, as the probe is placed away from the location of chest compressions and cardiac defibrillator pads. The PSL view is an excellent view for assessing qualitative cardiac activity, especially if SX views are inadequate, but is more difficult to obtain if the view is obstructed by ongoing chest compressions either from a provider or a mechanical compression device. If a PSL view is obtained, wiping off the gel before compressions resume can help avoid provider injury and inefficient compressions. Depending on the placement of defibrillator pads, an apical 4-chamber (A4C) view can also be used if the other views are challenging or unobtainable.
Recent data shows that obtaining transthoracic images during cardiac arrest can prolong pulse check duration. [12] Several strategies can be employed to ensure that the pulse check duration is not prolonged with the use of transthoracic echocardiography. First, the most experienced operator should perform the scan and only one sonographic window per pulse check is recommended.[6] Ideally, this provider’s primary focus should be on obtaining ultrasound images, with a second provider leading the resuscitation. The clip length on the ultrasound machine should be preset to less than 10 seconds to ensure that the duration of each ultrasound clip does not go beyond the 10 second pulse check period.
The ultrasound operator can attempt to find a sonographic window while compressions are ongoing to maximize success in recording images during the pulse check. This practice has been shown to decrease CPR pause length and ultrasound image acquisition time. [12] Once compressions are held during the pulse check, images can then be recorded. After the clip is recorded and pulse check is performed, chest compressions can resume. During chest compressions, the clip can be carefully interpreted without prolonging the pulse check duration (Figure 1).
Several structured protocols for use of ultrasound in cardiac arrest have been developed, including the cardiac arrest sonographic assessment (CASA) exam. [13] This algorithm runs as follows: at the first pulse check, the operator evaluates for the presence of cardiac tamponade; at the second pulse check, the operator evaluates for the presence of right heart strain, suggestive of a massive pulmonary embolism; lastly, at the third pulse check, the operator evaluates for the presence of cardiac activity. [13] While compressions are ongoing, operators may also assess for pneumothorax or for intraabdominal free fluid (eg, FAST exam), as needed. A follow up study by Clattenburg et al., demonstrated that implementation of this protocol significantly reduced the duration of chest compression interruptions—though, even with the CASA exam, pulse checks were still longer than without the use of POCUS. [14] This study highlights the benefit and need for continued education centered on algorithmic, standardized approaches to the use of ultrasound in the setting of cardiac arrest.
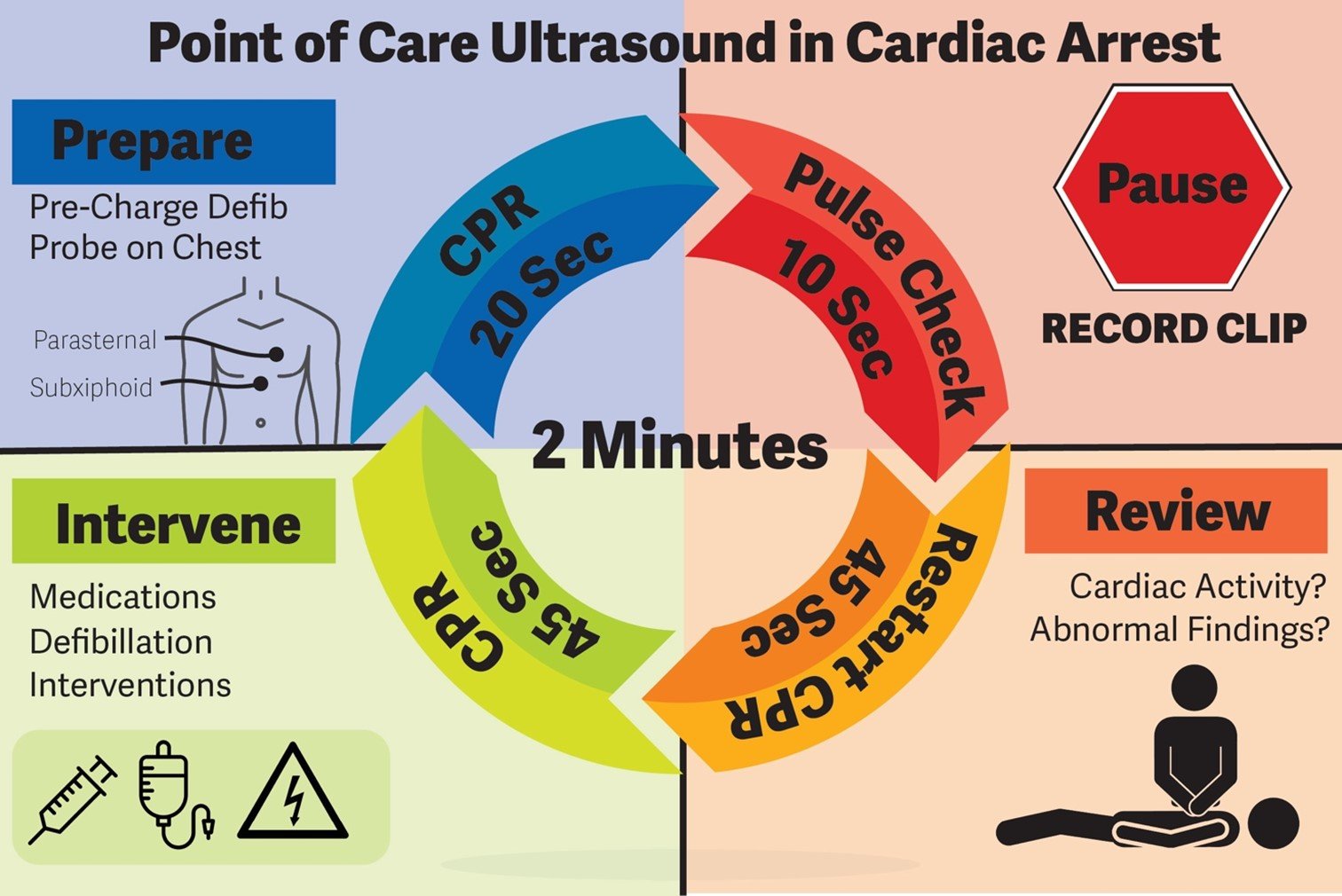
Figure 1. Protocol for Point of Care Ultrasound use in Cardiac Arrest
What to look for
Definitions of cardiac activity and standstill vary across the literature. [14] Most definitions describe ventricular contractility and a decrease in left ventricular chamber size, though discrepancies exist with the identification of atrial or valve flutter. Ultrasound operators may also mistake movement of the chest structures with mechanical ventilation as cardiac activity. Furthermore, weak myocardial contraction, profound bradycardia, or fine ventricular fibrillation can also be confused as cardiac standstill. Examples of cardiac standstill and a weak, bradycardic rhythm are highlighted in Figure 2.
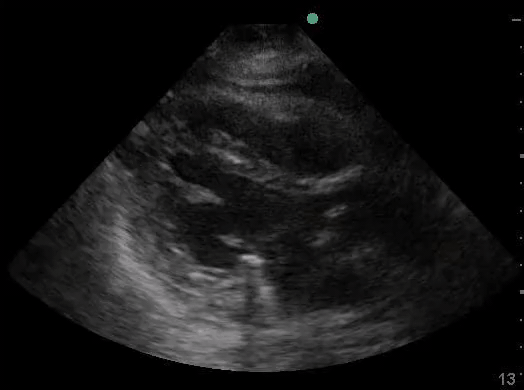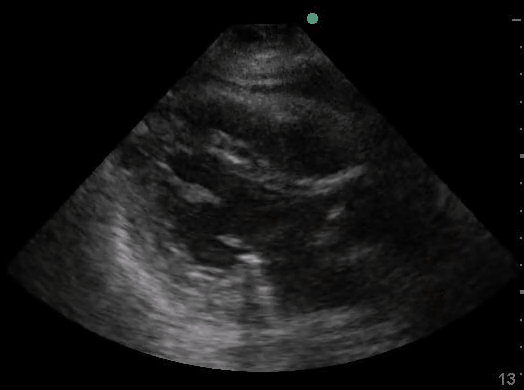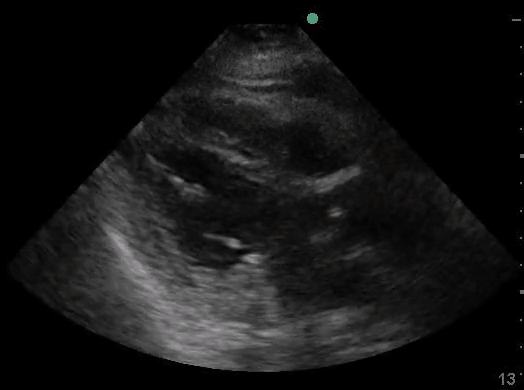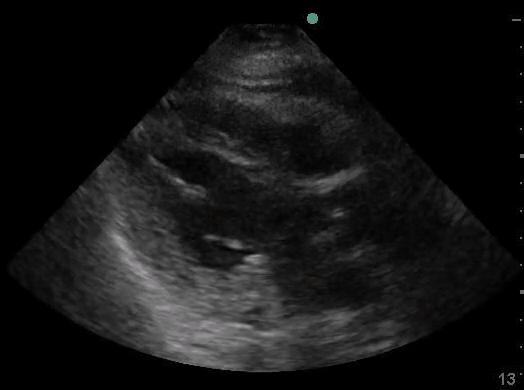
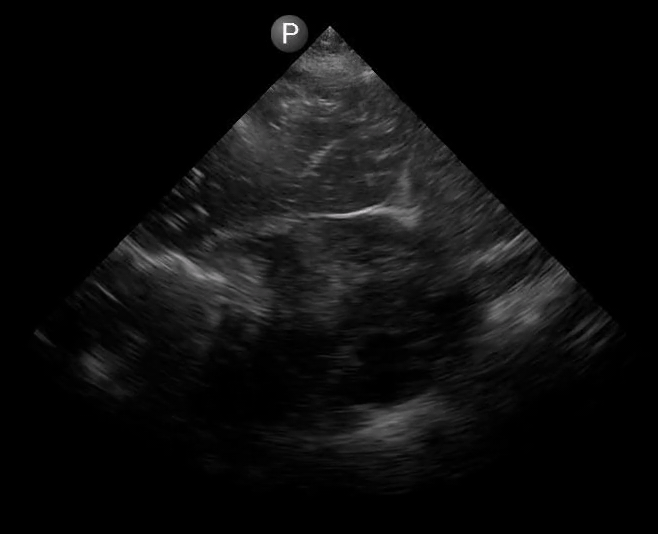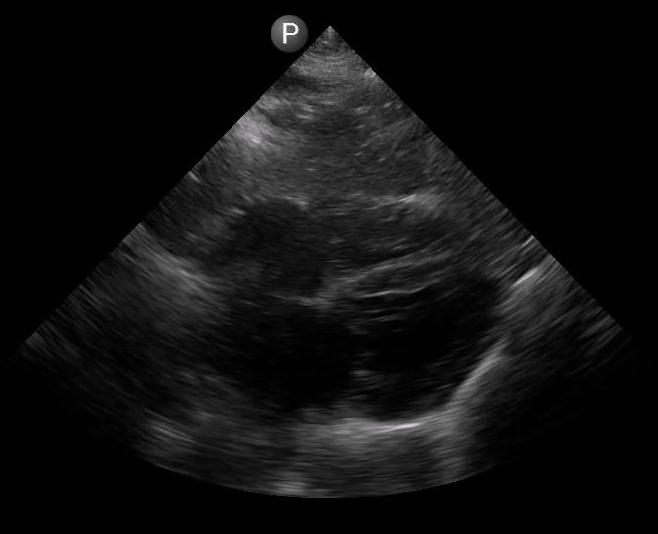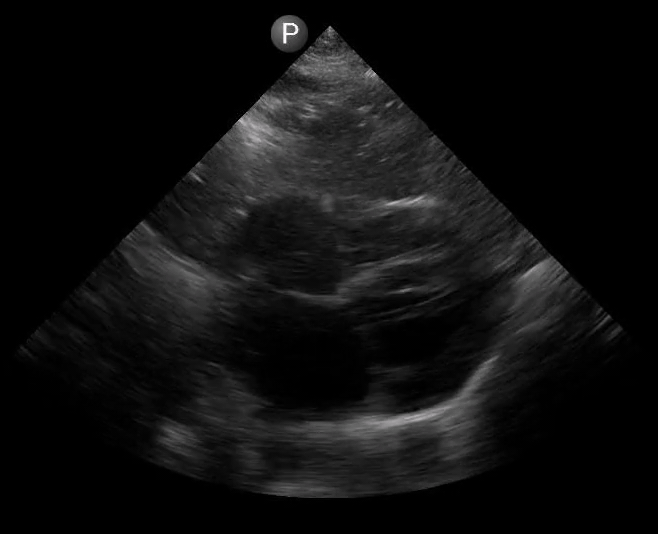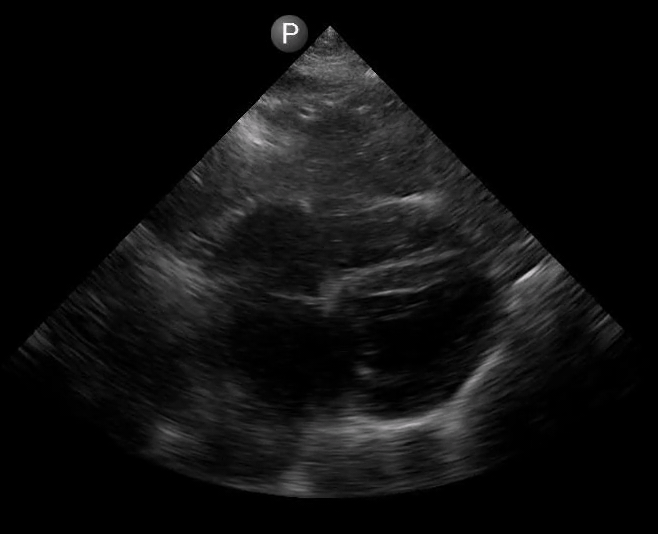
Figure 2. A) Parasternal long view with cardiac standstill. B) Subxiphoid view highlighting weak cardiac activity.
While investigating cardiac activity is a top priority during cardiac arrest, certain reversible causes of cardiac arrest, such as tamponade physiology from pericardial effusion or right heart strain from massive pulmonary embolism, can be appreciated on the recorded scan. Because review of the ultrasound image takes place while compressions are ongoing, ultrasound operators can take time to carefully look beyond the left ventricle for other cardiac findings. Procedures like pericardiocentesis or tPA could be initiated, if needed.
Additionally, the presence of a hemodynamically significant pneumothorax or pleural effusion, free fluid from an abdominal catastrophe, or an enlarged aorta can be appreciated on ultrasound. Depending on the length and direction of resuscitation, ultrasound operators may have an opportunity to find pathology due to intervenable, non-cardiac causes of cardiac arrest which may lead to expedited life-saving treatment.
Future Directions
Point-of-care transesophageal echocardiography (TEE) is becoming more widely used in cardiac arrest. It has four main advantages over TTE: a reliable sonographic window, continuous imaging of the heart during resuscitation, elimination of pauses in chest compressions, and potential to detect abnormalities that are more difficult to visualize on TTE. [15] However, barriers exist to widespread implementation of TEE in the ED, namely access to training, the cost of purchasing, storing, and reprocessing equipment. More research highlighting the benefits of point-of-care TEE is needed, and access to equipment and training opportunities would be greatly beneficial to the emergency medicine community.
Additionally, not all clinical environments have multiple providers available to have a dedicated provider perform the ultrasound during pulse checks of a resuscitation. If future machine learning algorithms could identify the presence or absence of cardiac activity, this would significantly reduce the cognitive load on a single provider leading a resuscitation. As AI technology advances in point-of-care ultrasound, there is exciting potential for improvement in the accuracy of cardiac activity detection.
Conclusion
Bedside echocardiography adds value to the diagnostic, prognostic, and treatment approach to patients in cardiac arrest. A protocolized approach can optimize resuscitation efforts. Ultrasound operators can optimize pulse check time by focusing on obtaining ultrasound images pre-pulse check and immediately encourage the resuscitation team to resume CPR once a clip is recorded. The clip can be interpreted for ventricular motion and reversible causes while CPR has resumed. While controversy exists on what cardiac standstill looks like, it does portend poor survival in cardiac arrest patients. As with much of the care of critically ill or injured patients, ultrasound provides tremendous information to aid in decision making during cardiac arrest.
Acknowledgements
The authors would like to acknowledge Sara Damewood, MD for her review and for providing the cardiac standstill image seen in Figure 2. The authors also thank John Collins for assisting with the creation and editing of Figure 1. Last, the authors would like to thank Jessica Schmidt, MD, MPH and Dana Resop, MD, for their thoughtful review and feedback.
References
[1] Gaspari R, Weekes A, Adhikari S, Noble VE, Nomura JT, Theodoro D, et al. Emergency department point-of-care ultrasound in out-of-hospital and in-ED cardiac arrest. Resuscitation. 2016;109:33-9. https://doi.org/10.1016/j.resuscitation.2016.09.018.
[2] Weekes AJ, Thacker G, Troha D, Johnson AK, Chanler-Berat J, Norton HJ, et al. Diagnostic accuracy of right ventricular dysfunction markers in normotensive emergency department patients with acute pulmonary embolism. Ann Emerg Med. 2016;68:277-91. https://doi.org/10.1016/j.annemergmed.2016.01.027.
[3] Alpert EA, Amit U, Guranda L, Mahagna R, Grossman SA, Bentancur A. Emergency department point-of-care ultrasonography improves time to pericardiocentesis for clinically significant effusions. Clin Exp Emerg Med. 2017;4:128–32. https://doi.org/10.15441/ceem.16.169.
[4] Salen P, Melniker L, Chooljian C, Rose JS, Alteveer J, Reed J, et al. Does the presence or absence of sonographically identified cardiac activity predict resuscitation outcomes of cardiac arrest patients? Am J Emerg Med. 2005;23:459-62. https://doi.org/10.1016/j.ajem.2004.11.007.
[5] Blaivas M, Fox JC. Outcome in Cardiac Arrest Patients Found to Have Cardiac Standstill on the Bedside Emergency Department Echocardiogram. Acad Emerg Med 2001;8:616-21. https://doi.org/10.1111/j.1553-2712.2001.tb00174.x.
[6] Hussein L, Rehman MA, Sajid R, Annajjar F, Al-Janabi T. Bedside ultrasound in cardiac standstill: a clinical review. Ultrasound J. 2019;11:35. https://doi.org/10.1186/s13089-019-0150-7.
[7] Tibballs J, Russell P. Reliability of pulse palpation by healthcare personnel to diagnose paediatric cardiac arrest. Resuscitation. 2009;80:61-4. https://doi.org/10.1016/j.resuscitation.2008.10.002.
[8] Zengin S, Gümüşboğa H, Sabak M, Eren ŞH, Altunbas G, Al B. Comparison of manual pulse palpation, cardiac ultrasonography and Doppler ultrasonography to check the pulse in cardiopulmonary arrest patients. Resuscitation. 2018;133:59-64. https://doi.org/10.1016/j.resuscitation.2018.09.018.
[9] Tayal VS, Kline JA. Emergency echocardiography to detect pericardial effusion in patients in PEA and near-PEA states. Resuscitation. 2003;59:315-8. https://doi.org/10.1016/S0300-9572(03)00245-4.
[10] Rabjohns J, Quan T, Boniface K, Pourmand A. Pseudo-pulseless electrical activity in the emergency department, an evidence based approach. Am J Emerg Med. 2020;38:371-5. https://doi.org/10.1016/j.ajem.2019.158503.
[11] Gaspari R, Weekes A, Adhikari S, Noble V, Nomura JT, Theodoro D, et al. A retrospective study of pulseless electrical activity, bedside ultrasound identifies interventions during resuscitation associated with improved survival to hospital admission. A REASON Study. Resuscitation. 2017;120:103-7. https://doi.org/10.1016/j.resuscitation.2017.09.008.
[12] Gaspari R, Harvey J, DiCroce C, Nalbandian A, Hill M, Lindsay R, et al. Echocardiographic pre-pause imaging and identifying the acoustic window during CPR reduces CPR pause time during ACLS – A prospective Cohort Study. Resuscitation Plus. 2021;6:100094. https://doi.org/10.1016/j.resplu.2021.100094.
[13] Gardner KF, Clattenburg EJ, Wroe P, Singh A, Mantuani D, Nagdev A. The Cardiac Arrest Sonographic Assessment (CASA) exam – A standardized approach to the use of ultrasound in PEA. Am J Emerg Med. 2018;36:729–31. https://doi.org/10.1016/j.ajem.2017.08.052.
[14] Clattenburg EJ, Wroe PC, Gardner K, Schultz C, Gelber J, Singh A, et al. Implementation of the Cardiac Arrest Sonographic Assessment (CASA) protocol for patients with cardiac arrest is associated with shorter CPR pulse checks. Resuscitation. 2018;131:69–73. https://doi.org/10.1016/j.resuscitation.2018.07.030.
[15] Blaivas M. Transesophageal echocardiography during cardiopulmonary arrest in the emergency department. Resuscitation. 2008;78:135–40. https://doi.org/10.1016/j.resuscitation.2008.02.021.



