Cardiac Tamponade and Ultrasound-Guided Pericardiocentesis
Xiangyun J Duan, MD, PGY2 Emergency Medicine Resident, University of Wisconsin-Madison
Hani Kuttab, MD, Assistant Professor; Assistant Ultrasound Director, University of Wisconsin-Madison
Introduction
Cardiac tamponade is an uncommon yet important diagnosis to make in the emergency department (ED). It results from an acute or subacute pericardial effusion accumulating under pressure, which prevents proper ventricular relaxation and filling during diastole. This reduces cardiac output and precipitates obstructive shock and subsequent cardiovascular collapse. Etiologies of pericardial effusions and tamponade include infection, uremia, malignancy, autoimmune disease, and post-myocardial infarction.1-4 The ability to recognize cardiac tamponade and promptly intervene with emergent pericardiocentesis is a key skill of emergency physicians. Timely and accurate diagnosis is crucial. Point-of-care echocardiography plays a key role in both diagnosis and intervention. Emergent pericardiocentesis may be a life-saving procedure and is indicated in patients with pericardial effusions with associated hemodynamic instability.
Diagnosis
Historically, cardiac tamponade was diagnosed as a constellation of clinical findings, including Beck’s triad (hypotension, jugular venous distention, muffled heart sounds), pulsus paradoxus, and electrical alternans on electrocardiography. However, studies have demonstrated that the clinical history and physical exam perform poorly, with the sensitivity of Beck’s triad being 0% in one review.5 Furthermore, vital sign derangements are also not reliable predictors of tamponade; tachycardia was only present in 59.5% of patients, while hypotension was only present in 31.4%.5 Thus, point-of-care echocardiography should be used to confirm the presence of pericardial effusions and secure a diagnosis of tamponade. Although trivial and small effusions may be difficult to visualize, moderate to large pericardial effusions are easily identified as fluid collections surrounding the myocardium in the subxiphoid view. On parasternal long views, pericardial effusions may be visualized anterior to the right ventricular free wall and posterior to the LV, crossing in front of the descending aorta (Figure 1). While serous pericardial effusions are typically hypoechoic and readily identified as a fluid-filled space, hemorrhagic or purulent effusions may be heterogeneous and similar in appearance to a generous pericardial fat pad, although fluid motion is likely to be seen.4
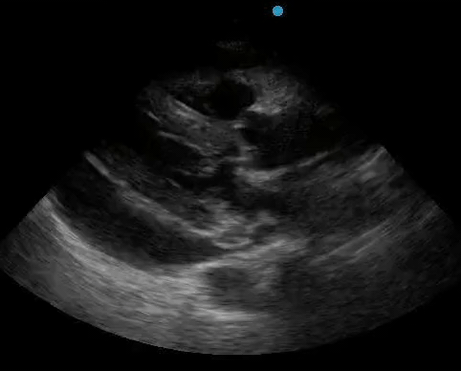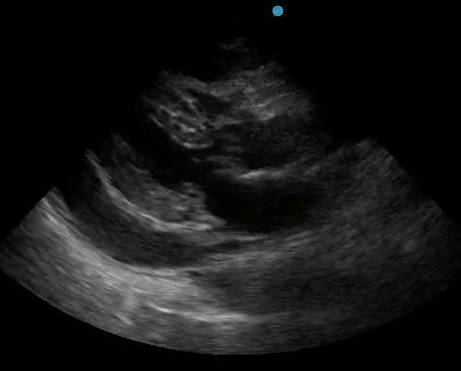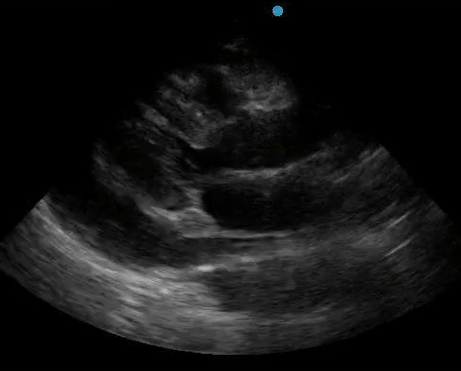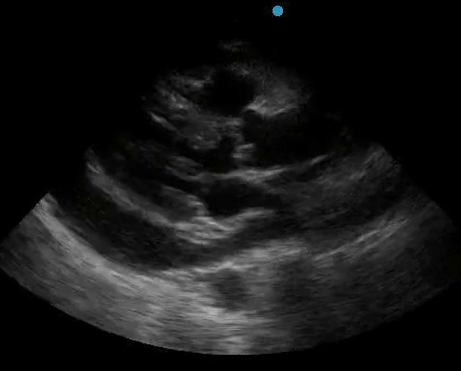
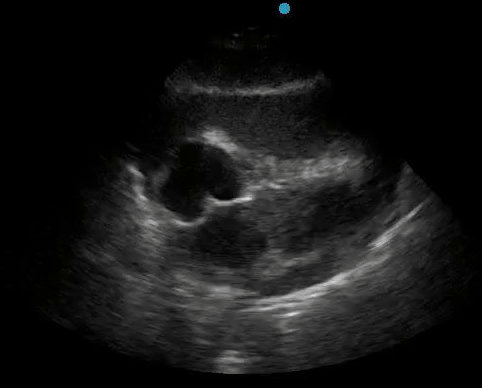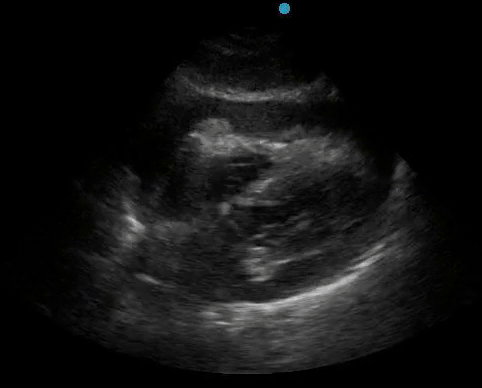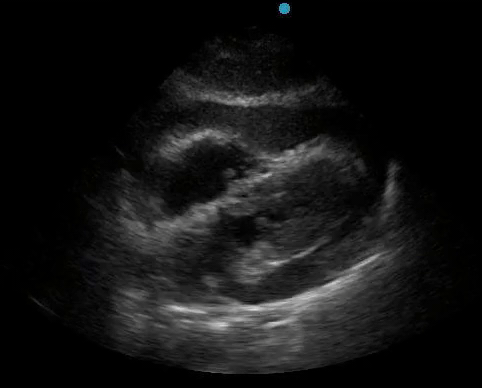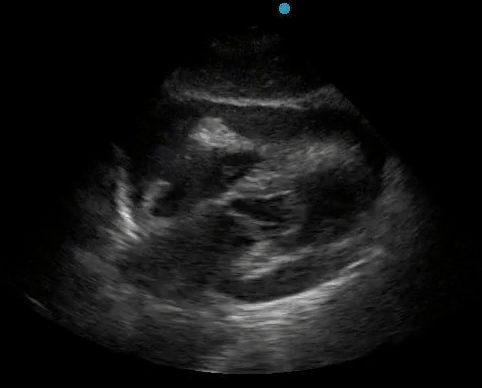
Figure 1. A) Subxiphoid cardiac view, demonstrating a moderately-sized pericardial effusion. B) Parasternal Long Axis cardiac view, demonstrating a moderately-sized pericardial effusion which tracks anterior to the descending aorta.
Hallmarks of cardiac tamponade on ultrasound include:
- Presence of a pericardial effusion
- Diastolic right ventricular collapse (more than 90% specific fortamponade)
- Right atrial collapse during systole (lasting greater than 1/3 of systole)
- Plethoric inferior vena cava
- Significant variation in mitral and tricuspid inflow velocity by pulse wave doppler from the apical view (sonographic pulsus paradoxus).1
It is important to note that tamponade can occur with varying degrees of pericardial effusions and that the size of the effusion itself is not diagnostic for tamponade.
Ultrasound-Guided Pericardiocentesis Technique
Resuscitation
In general, procedural intervention is the standard of care for cardiac tamponade. However, given that right ventricular filling is preload dependent in the setting of high intrapericardial pressure, fluid resuscitation is reasonable if the patient shows signs of intravascular hypovolemia. Consider the addition of inotropes if suspicious for concurrent cardiogenic shock. Last, ensure that the patient has the sonographic signatures of pericardial tamponade prior to beginning the procedure, as outlined above.
Materials
Pericardiocentesis kits are available and include the relevant items for access and for placing a pericardial drain by the Seldinger technique. If a specific pericardiocentesis kit is not available, other materials gathered in the ED may be used, including an 18-gauge spinal needle, a J-tipped wire, a small-bore catheter, and a three-way stopcock, and a bulb or gravity-dependent drain. Many of these supplies can be located in a traditional central line kit, with the exception of the spinal needle.
Needle Guidance
Two techniques have been described for ultrasound guidance. First, a high-frequency probe may be included in the sterile field and used by the operator to directly visualize the needle as it approaches and enters the pericardium. A needle guide would provide ideal visualization and control of the needle as it advances. This technique provides superior control during access to the pericardium. Alternatively, a second clinician may provide constant visualization from an adjacent traditional echocardiography view to identify the needle and confirm entrance to the pericardium. The phased array or curvilinear probes are used, although visualization of the needle is much more challenging with the phased array probe. This technique requires the operator to memorize the expected needle trajectory and depth of pericardium at that site but provides superior real-time surveillance of cardiac function as pericardiocentesis progresses.3-6
With either technique, a small volume of agitated saline injected into the pericardium could also provide sonographic contrast and confirm entrance into the pericardium. An ECG lead may also be attached to the needle to monitor for ST elevation, which indicates contact with the myocardium.
Selection of Approach
Ultrasound-guided pericardiocentesis is the standard of care when ultrasound is available and is preferred over the landmark-guided procedure. The heart and pericardial fluid are surveyed from standard transthoracic echocardiography views, and the maximum fluid pocket is identified. Depending on the patient's anatomy and the largest pocket of fluid identified under ultrasound, the ideal approach may be right parasternal, left parasternal, apical, or subxiphoid. However, the left parasternal and apical approaches are the most pursued and have been shown to be superior to the classic subxiphoid approach.6
Parasternal Approach
The parasternal approach may be advantageous due to the shallow intercostal tissue that must be traversed to reach the pericardium. The selection of laterality and intercostal space depends on individual patient anatomy. Similar to percutaneous tube thoracostomy, care must be taken to avoid laceration of the subcostal neurovascular bundle. There is a risk of laceration to the internal mammary artery, which courses approximately one centimeter lateral to the sternal border. Using the high-frequency linear probe, color Doppler can identify the artery when selecting a needle trajectory. Lastly, there are risks of pneumothorax and right ventricular lacerations with this approach.3-6
Apical Approach
The apical approach may provide a relatively low risk of a myocardial puncture due to the oblique angle of myocardial tissue at this approach. This approach may be complicated by the presence of substantial breast tissue, and care should be taken to retract any intervening breast tissue. Additionally, the presence of lung tissue both degrades visualization of the heart and increases the risk of pneumothorax, so an alternative approach should be considered if traversing the lung cannot be avoided. Lastly, there is a risk of right or left ventricular lacerations.3-6
Subxiphoid Approach
The subxiphoid approach requires traversing extensive deep tissue. The needle trajectory should be selected to minimize the amount of liver parenchyma traversed due to the possibility of hepatic laceration and hemorrhage. Additionally, sitting the patient upright at 30 degrees may allow for the pericardial fluid to layer at the bottom portion of the heart and increase the pocket size for needle entry. Though there is a relative absence of critical structures compared to the above approaches, there is an associated risk of liver laceration or puncture with this approach.3-6
General Technique
Once an approach and needle trajectory are selected, optimize patient and ultrasound machine position. Create a sterile field and confirm that the necessary supplies are present. Fill the syringe with a local anesthetic and load the needle. Confirm satisfaction with the planned needle trajectory and adequate ultrasound probe placement for guidance. Anesthetize the skin and enter the trajectory. Furthermore, conscious sedation could be considered to minimize patient movement during the procedure.
Advance the needle slowly in 1-2mm steps while drawing negative pressure and under direct ultrasound guidance. It is advised to restrict the amount of back-and-forth motion with the needle (eg, do not bounce the needle within the body to identify it under ultrasound). Practice moving the ultrasound probe carefully to locate and identify the needle tip. And, as always, do not advance your needle unless you see the needle tip.
Once the pericardial fluid is aspirated, advance 1-2mm again and confirm the proper position of the needle tip with ultrasound. This is achieved by direct sonographic visualization of the needle tip or by instillation and sonographic visualization of agitated saline. Begin aspirating pericardial fluid. Depending on the chronicity of the patient’s effusion, removal of as little as 10mL of fluid may be sufficient to temporize the patient’s hemodynamics. Inspect the pericardial fluid. The presence of a clot indicates acute hemorrhage or ventricular puncture. Hemorrhagic, purulent, or serous appearance may guide resuscitation and management.
Once tamponade has been relieved and hemodynamic stability has been restored, repeat point-of-care echocardiography and document resolution of signs of tamponade. Additionally, evaluation for lung sliding should be completed to assess for pneumothorax. The procedure may be concluded at this point, although clinicians familiar and comfortable with the Seldinger technique may elect to place a pericardial drain. Serous effusions typically require multiple days of drainage and drain placement may obviate recurrent tamponade and the need for repeat pericardiocentesis. The total volume of fluid removed and whether to place a drain should be decided in consultation with cardiology. Otherwise, secure the pericardial drain and attach accessory drainage devices. Send fluid for chemical analysis, cytology, and culture as indicated.3-6
Contraindications
There is no absolute contraindication to pericardiocentesis since obstructive shock from cardiac tamponade is an acute life threat. However, extreme caution should be exercised if aortic dissection or rupture is suspected. Furthermore, relief of tamponade may exacerbate hemorrhage directly or by increasing arterial pressure. Coagulopathy or thrombocytopenia increases the risk of hematoma and hemorrhage, and a reversal may reduce periprocedural risk. Pulmonary hypertension increases the risk of hemodynamic collapse as a decompensated right ventricle may acutely fail in the face of fluctuating intrapericardial pressures.
Complications
Case series of pericardiocentesis performed by experienced cardiologists show a low complication rate on the order of 1%. Major complications include myocardial laceration and ventricular puncture, although immediate transventricular hemorrhage is atypical with a needle puncture. This complication should be discussed with cardiothoracic surgery in case emergent cardiac repair is necessary. Direct myocardial stimulation by procedure devices may induce arrhythmia. Failure to aspirate fluid or production of a smaller than expected volume of fluid may indicate a technical error or a complex loculated effusion. Pericardiocentesis may be attempted again from a different approach, or if hemodynamics improves sufficiently, transfer to a surgical or interventional setting may be considered.
Conclusions
Cardiac tamponade is important, although uncommon etiology of shock. Point-of-care echocardiography plays a key role in confirming the diagnosis, as well as in optimizing first-pass success and complication rates of pericardiocentesis.
References
- Alerhand S, Carter JM. What echocardiographic findings suggest a pericardial effusion is causing tamponade? Am J Emerg Med. 2019;37(2):321-6.
- Flint N, Siegel RJ. Echo-Guided Pericardiocentesis: When and How Should It Be Performed? Curr Cardiol Rep. 2020;22(8):71.
- Luis SA, Kane GC, Luis CR, Oh JK, Sinak LJ. Overview of Optimal Techniques for Pericardiocentesis in Contemporary Practice. Curr Cardiol Rep. 2020;22(8):60.
- Vakamudi S, Ho N, Cremer PC. Pericardial Effusions: Causes, Diagnosis, and Management. Prog Cardiovasc Dis. 2017;59(4):380-8.
- Stolz L, Valenzuela J, Situ-LaCasse E, et al. Clinical and historical features of emergency department patients with pericardial effusions. World J Emerg Med. 2017;8(1):29.
- Stolz L, Situ-LaCasse E, Acuña J, et al. What is the ideal approach for emergent pericardiocentesis using point-of-care ultrasound guidance? World J Emerg Med. 2021;12(3):169.



