Motion of the HOCean: A Clinical Case of Hypertrophic Obstructive Cardiomyopathy (HOCM)
Kolten Fischer, MD, Virginia Commonwealth University
Lindsay Taylor, MD, FACEP, Virginia Commonwealth University
Case Presentation
An otherwise healthy 18-year-old male presents to the emergency department (ED) via ambulance after a syncopal episode while playing basketball this morning. He states he became dizzy while running up the court and woke up surrounded by his coach and teammates. He reports this is the second syncopal episode he has suffered while playing basketball this month.
On arrival, the patient’s vital signs include a temperature of 36.9° C, BP 117/76, HR 71 and SpO2 100% on room air. The examination shows an athletic-appearing male in no acute distress and a cardiac exam with a systolic murmur heard at the left lower sternal border.
Initial workup showed an electrocardiogram (Figure 1) with left ventricular hypertrophy (LVH) and Q waves in the lateral leads.
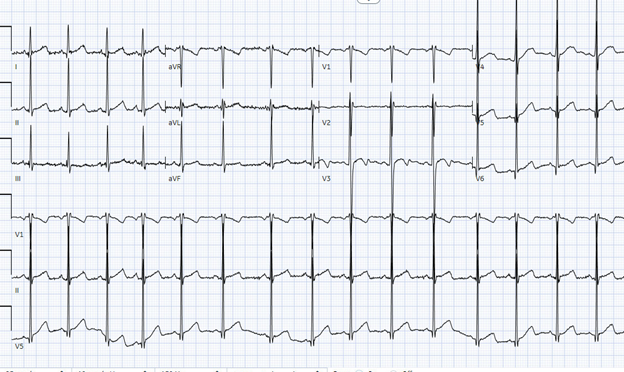
Figure 1
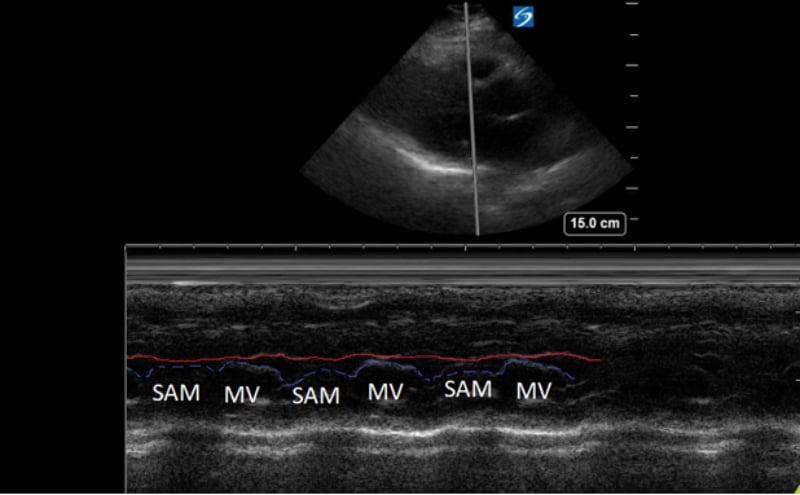
A point-of-care ultrasound was performed to evaluate for potential etiologies of syncope. The ultrasound displayed biventricular and interventricular septal hypertrophy (Figure 2), systolic anterior motion of the mitral valve (SAM, Figure 3), and a “dagger-like” continuous wave Doppler waveform emerging from the left ventricular outflow tract. (LVOT, Figure 4)
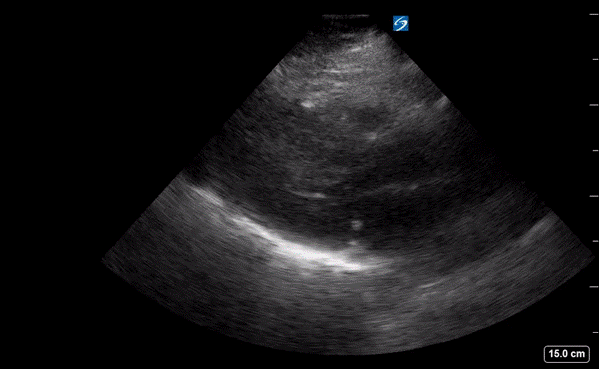
Figure 2. Parasternal long-axis view displaying a hypertrophic left ventricle (LV) and right ventricle (RV).
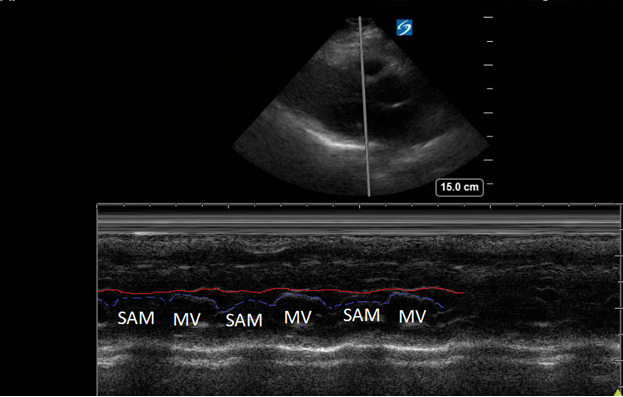
Figure 3. Parasternal long-axis view using M-Mode displaying SAM of the mitral valve against the interventricular septum (red line). Note how the mitral valve shifts anteriorly during the same period that the septum contracts posteriorly.
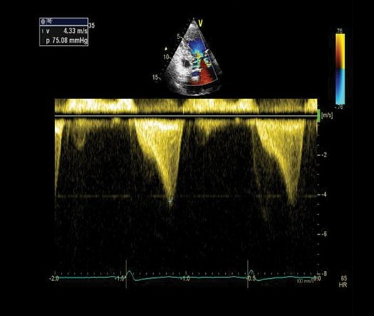
Figure 4. Apical 5-chamber view using continuous wave Doppler displaying a “dagger-like” left ventricular outflow obstruction with a maximal peak gradient of 75 mmHg.1
Background
Hypertrophic cardiomyopathy (HCM) is an inherited disease caused by various mutations in the sarcomere genes which encode the structural apparatus of the heart musculature. It is prevalent in approximately one out of every 200-500 members of the general population.2,3 Notably, these patients are at an increased incidence of sudden mortality events such as ventricular tachycardia, ventricular fibrillation or sudden cardiac deaths occurring in 0.5-1.5% per year in adults and 2% in children, adolescents, and young adults.4 It is one of the most common causes of sudden cardiac death in children and young adults, and athletes, such as in our patient.5
HCM is diagnosed using ultrasound with the below criteria:
1) Unexplained wall thickness (no history of longstanding hypertension, aortic stenosis, high level athlete, etc.) > 15 mm in any myocardial segment during diastole
2) Asymmetric septal hypertrophy. Interventricular septum/posterior wall thickness ratio of:
a. >1.3 in normotensive patients
b. >1.5 in hypertensive patients1
Hypertrophic obstructive cardiomyopathy (HOCM) refers to the common obstructive subset that affects 70% of patients with HCM.6 Left ventricular outflow tract obstruction (LVOTO) has been shown to be an independent predictor for adverse outcomes by multiple studies.7 Traditionally, systolic anterior motion (SAM) of the mitral valve was thought to be pathognomonic for HCM. Recent studies have shown that of the HCM patients with SAM, 25-50% will have LVOTO.8 Using ultrasound, SAM is observed in the parasternal long-axis using M-mode over the anterior leaflet of the mitral valve. The time the valve is contact with the septum quantifies disease severity.
1) Mild: brief SAM without septal contact
2) Moderate: septal contact < 1/3 of systolic period
3) Severe: septal contact > 1/3 of systolic period9
Additionally, patients who have SAM with LVOTO have concurrent mitral regurgitation. The regurgitant jet is predominantly posterior. An anteriorly oriented regurgitant jet points to a primary mitral valve disease versus SAM as the etiology of the mitral regurgitation.10
The left ventricular outflow tract (LVOT) gradient is another way bedside ultrasound can be utilized in the diagnosis of obstructive HCM. This is performed by placing the continuous wave Doppler beam over the LVOT in an apical 5-chamber view. A gradient > 30 mmHg is defined as intraventricular obstruction. About 1/3 of patients with HCM have gradients > 30 mmHg at rest, another 1/3 have it with provocation (Valsalva/exercise/medication), and 1/3 have no obstruction.11 In the ED, a Valsalva maneuver is an imperfect (underestimates exercise-induced obstruction) but convenient provocation. Furthermore, a gradient of > 50 mmHg is considered hemodynamically significant, often requiring invasive septal reduction therapies.12 Continuous Doppler images will classically show a “dagger-like,” late-to-rise waveform characteristic of HCM.
Case Resolution
Our patient was seen and evaluated by the Cardiology service in the emergency department. He was admitted to the Cardiology service for a diagnostic echocardiogram and medication optimization with beta-blockers. The patient was discharged two days later with outpatient cardiology follow up and activity restrictions.
Series
ACEP’s critical care ultrasound subcommittee has been working on advanced cardiac ultrasound educational cards to share with the community as a reference when performing critical care bedside ultrasound. Figure 5 is a graphical representation of HCM and the pathologic measurements. It is a useful tool when discussing a case with your consultants. Stay tuned for more to come from the group!
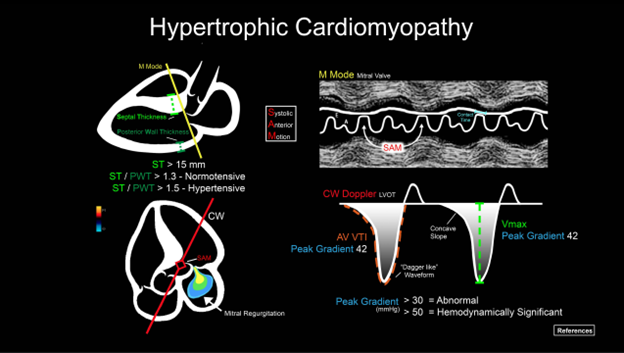
Figure 5. Illustration of an advanced cardiac ultrasound educational card on aortic stenosis.
References
- Cardim N, Galderisi M, Edvardsen T, et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur Heart J Cardiovasc Imaging. 2015;16(3):280.
- Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92(4):785-9. doi: 10.1161/01.cir.92.4.785. PMID: 7641357.
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-54. doi: 10.1016/j.jacc.2015.01.019. PMID: 25814232.
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy. Lancet. 2017;389(10075):1253-1267. doi: 10.1016/S0140-6736(16)31321-6. Epub 2016 Nov 30. Erratum in: Lancet. 2017;389(10075):1194. PMID: 27912983.
- Maron BJ, Haas TS, Ahluwalia A, Murphy CJ, Garberich RF. Demographics and epidemiology of sudden deaths in young competitive athletes: From the United States National Registry. Am J Med. 2016;129(11):1170-7. doi: 10.1016/j.amjmed.2016.02.031. Epub 2016 Apr 1. PMID: 27039955.
- Prinz C, Farr M, Hering D, Horstkotte D, Faber L. The diagnosis and treatment of hypertrophic cardiomyopathy. Dtsch Arztebl Int. 2011;108(13):209-15. doi: 10.3238/arztebl.2011.0209. Epub 2011 Apr 1. PMID: 21505608; PMCID: PMC3078548.
- Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348(4):295-303. doi: 10.1056/NEJMoa021332. PMID: 12540642.
- Ibrahim M, Rao C, Ashrafian H, Chaudhry U, Darzi A, Athanasiou T. Modern management of systolic anterior motion of the mitral valve. Eur J Cardiothorac Surg. 2012;41(6):1260-70. doi: 10.1093/ejcts/ezr232. Epub 2012 Jan 18. PMID: 22290892.
- Guigui SA, Torres C, Escolar E, Mihos CG. Systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy: a narrative review. J Thorac Dis. 2022;14(6):2309-2325. doi: 10.21037/jtd-22-182. PMID: 35813751; PMCID: PMC9264047.
- Yeo TC, Miller FA Jr, Oh JK, Schaff HV, Weissler AM, Seward JB. Hypertrophic cardiomyopathy with obstruction: important diagnostic clue provided by the direction of the mitral regurgitation jet. J Am Soc Echocardiogr. 1998;11(1):61-5. doi: 10.1016/s0894-7317(98)70121-x. PMID: 9487471.
- Williams LK, Frenneaux MP, Steeds RP. Echocardiography in hypertrophic cardiomyopathy diagnosis, prognosis, and role in management. Eur J Echocardiogr. 2009;10(8):iii9-14. doi: 10.1093/ejechocard/jep157. PMID: 19889657.
- Kitai T, Xanthopoulos A, Nakagawa S, Ishii N, Amano M, Triposkiadis F, Izumi C. Contemporary diagnosis and management of hypertrophic cardiomyopathy: The role of echocardiography and multimodality imaging. J Cardiovasc Dev Dis. 2022;9(6):169. doi: 10.3390/jcdd9060169. PMID: 35735798; PMCID: PMC9224724.