ACEP ID:
- My Account
- My CME
- Sign Out
ACEP ID:
Chest x-rays and chest CTs are not useful to diagnose COVID-19 infection, since findings completely overlap with those of other pulmonary infections. These imaging studies should be ordered in COVID-19 patients using the same criteria for other pulmonary diseases (ie, to diagnose complications such as pneumothorax and emboli and to risk stratify sick patients). With that said, a chest x-ray may be unnecessary in the workup of a COVID-19 patient with mild disease. In addition, lung ultrasound is probably equally sensitive and specific for clinically significant infiltrates and pneumothorax. Ultrasound equipment is also easier to disinfect than x-ray and CT equipment.
For more information on the use of these imaging modalities in cases of COVID-19, refer to the American College of Radiology (ACR) “Recommendations for the Use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection.”
Facilities can consider deploying portable radiography units in ambulatory care facilities when chest x-rays are considered medically necessary. The surfaces of these machines can be cleaned easily, and using portable units avoids having to take infectious patients into radiography rooms, where they may spread infection. Chest x-ray findings are nonspecific and can be normal (Table 9.3).
Table 9.3 Characteristics of the radiographic findings reported by the NIH from 11 radiologists who re-read chest x-rays of COVID-19 patients seen in greater New York City urgent care centers from March 9 to March 24, 2020 (N = 636). Credit: Weinstock MB, Echenique A, Russell JW, et al. Chest x-ray findings in 636 ambulatory patients with COVID-19 presenting to an urgent care center: a normal chest x-ray is no guarantee. J Urgent Care Med. Published May 2020.
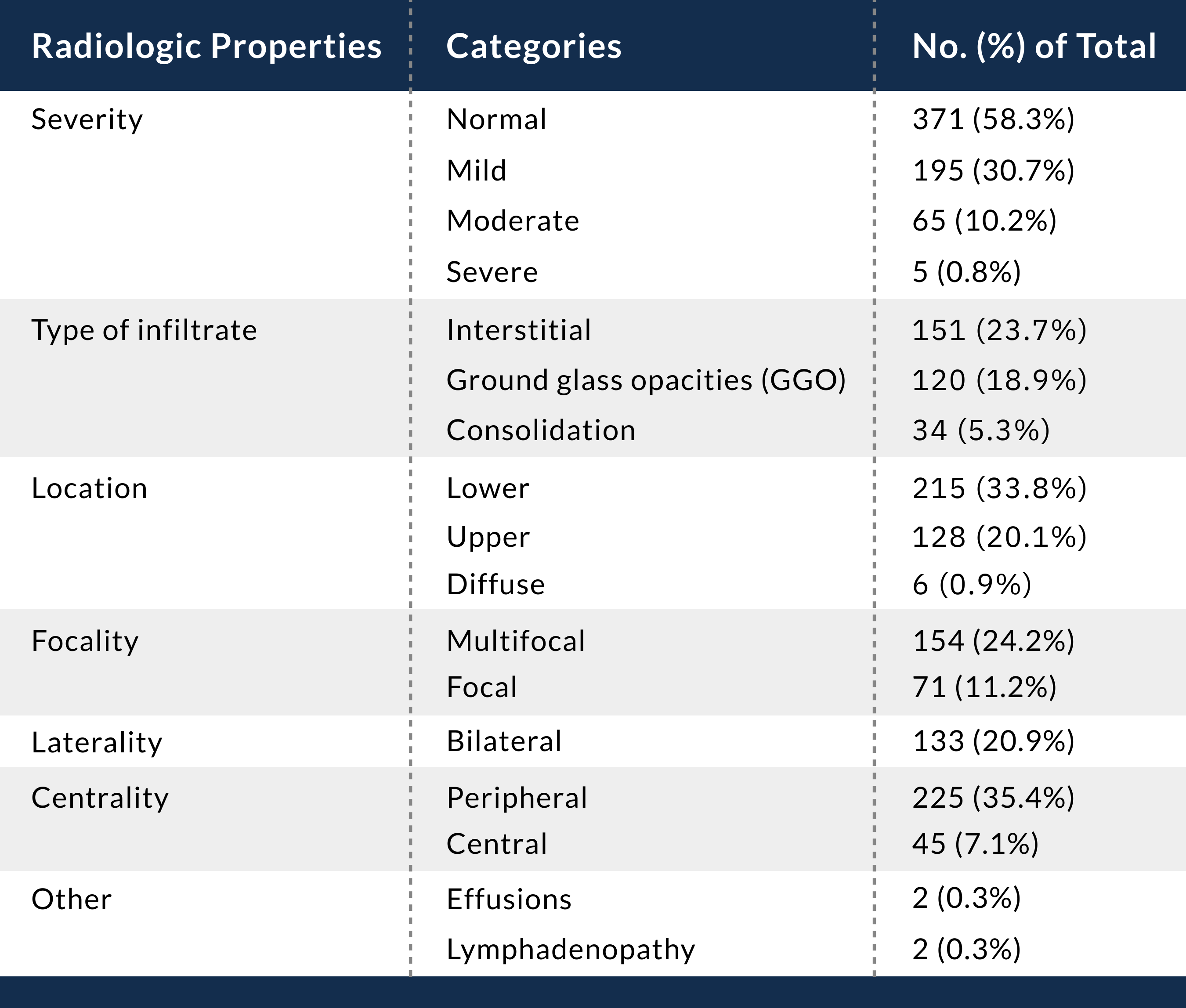
Note: Because some patients had more than one finding, numbers do not add to 100%.
Author: Christopher Sampson, MD, FACEP, Vice Chair-Research, University of Missouri School of Medicine
Based on the risks of misdiagnosis and viral transmission, the ACR recommends that CT not be used to screen for or as a first-line test to diagnose COVID-19. CT should be used sparingly and reserved for hospitalized, symptomatic patients with specific clinical indications. Appropriate infection control procedures should be followed before scanning subsequent patients.
The primary CT findings in patients with COVID-19 are ground-glass opacities (GGOs) and consolidation. Bilateral findings also appear to be more common. GGOs and opacities are more likely to be an intermediate or late finding in the disease process.1 Anecdotally, GGOs have been discovered incidentally in patients who received imaging for other clinical indications.
One study reported that there were more consolidated lung lesions in patients 5 days or more from disease onset and in patients older than 50 years.2