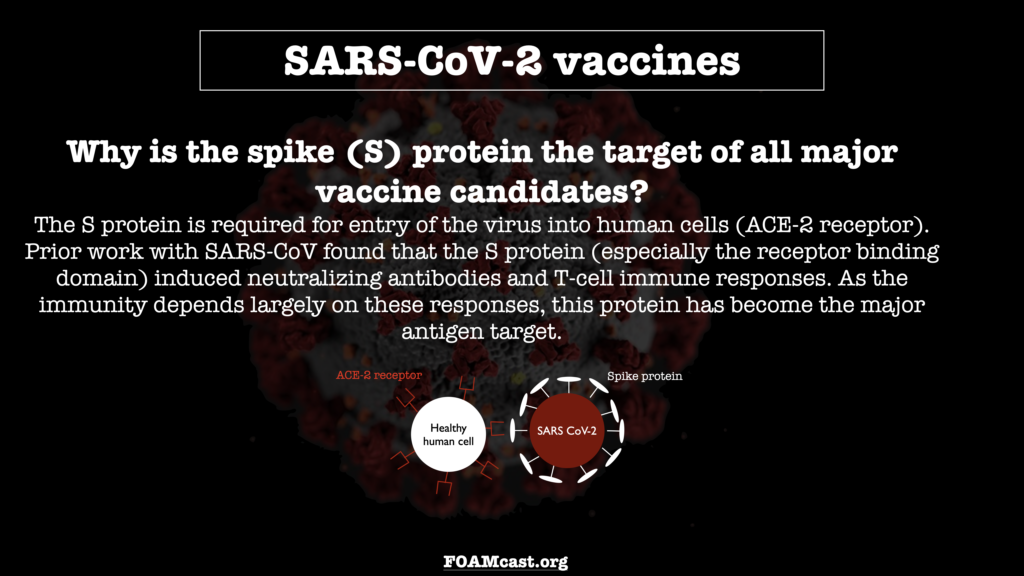
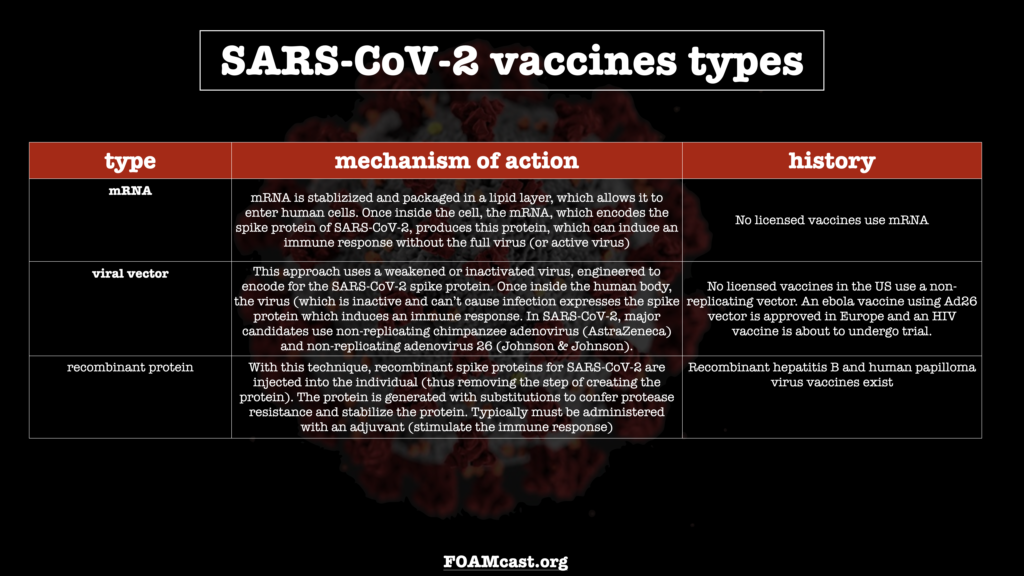
In this episode, we review what we know, as of November 29, 2020, about the major SARS-CoV-2 vaccine candidates – their mechanisms of action and preliminary data. Be aware that most of the data is preliminary and exact numbers may change (and long-term data is not yet available)
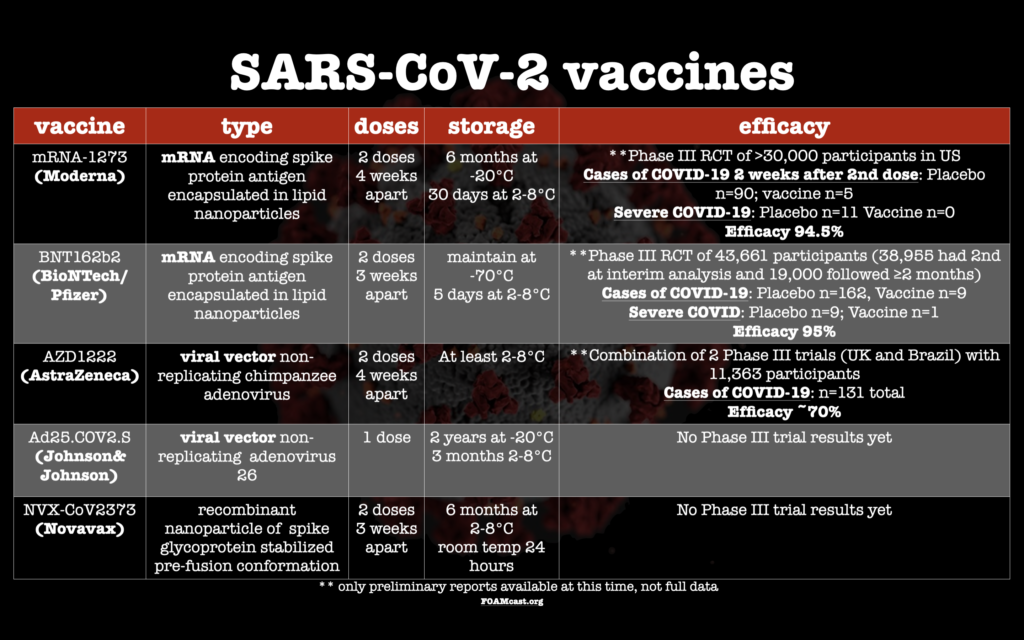
Moderna vaccine data
Phase I dose-escalation trial (Jackson et al NEJM 2020) and small 40 patient study of immunogenicity in older adults (Anderson et al NEJM 2020). (phase III study protocol )
Phase III trial results (Baden et al, NEJM)
- >30,000 participants within the US
- 37% from racial/ethnic minority groups (>6000 Hispanic participants, >3000 African American participants)
- 22% healthcare workers
- >14,000 female
- >7000 >65 years old
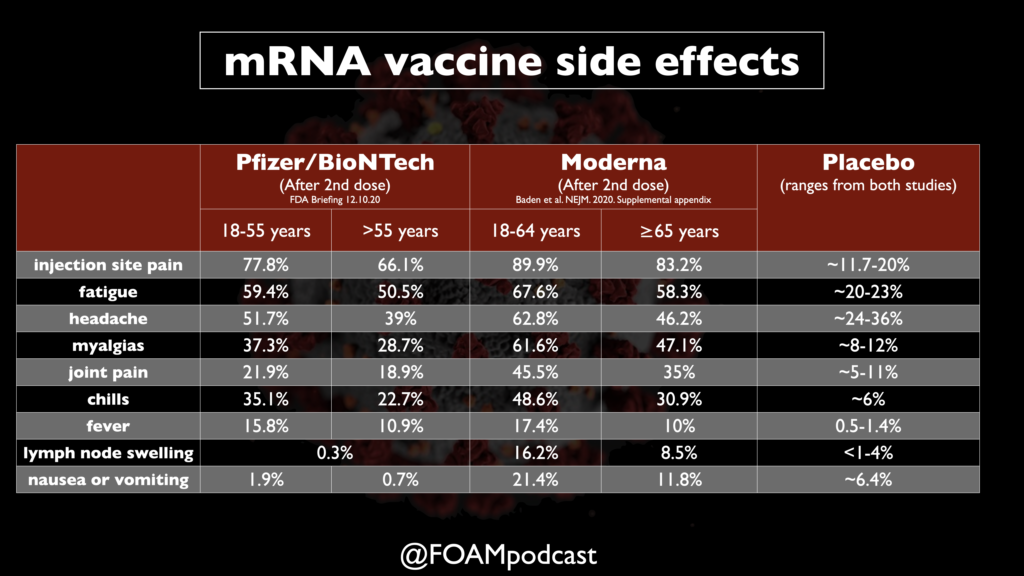
Pfizer/BioNTech vaccine data
Data from the FDA Emergency Use Authorization Briefing and Polack et al in NEJM
About 19,000 participants were followed for ≥2 months
- Looked at common side effects in 8,000 of the participants. Fatigue 3.7% after 2nd dose, headache 2%
AstraZeneca vaccine data
Phase I/II safety/immunogenicity study (Folegatti et al Lancet 2020)
Press Release data (11/23/20) from an interim (phase III trial protocol)
Lancet manuscript Phase 2/3 trial results
Johnson & Johnson vaccine data
Phase I/IIa safety/immunogenicity study (Sadoff et al MedRxiv.org [pre-print])
Novavax vaccine data